Grade 8 - Physical Science
advertisement

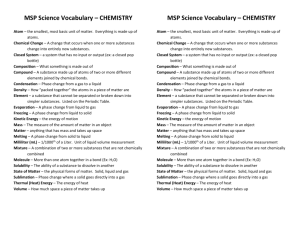
Unit Plan Agenda Science Content Area: Unit Plan Title: Matter Overview of Unit Grade(s) 8 All objects and substances in the natural world are composed of matter. Matter has two fundamental properties: matter takes up space, and matter has inertia. Essential Question(s) and Enduring Understandings (in grade appropriate terminology). 1. How do the properties of materials determine their use? The structures of materials determine their properties. 2. How does conservation of mass apply to the interaction of materials in a closed system? When materials interact within a closed system, the total mass of the system remains the same. Content Statements and CPI Content: All matter is made of atoms. Matter made of only one type of atom is called an element. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements. Content: All substances are composed of one or more of approximately 100 elements. 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” Content: Properties of solids, liquids, and gases are explained by a model of matter as composed of tiny particles (atoms) in motion. 5.2.8.A.3 Use the kinetic molecular model to predict how solids, liquids, and gases would behave under various physical circumstances, such as heating or cooling. Content: The Periodic Table organizes the elements into families of elements with similar properties. 5.2.8.A.4 Predict the physical and chemical properties of elements based on their positions on the Periodic Table. Content: Elements are a class of substances composed of a single kind of atom. Compounds are substances that are chemically formed and have physical and chemical properties that differ from the reacting substances 5.2.8.A.5 Identify unknown substances based on data regarding their physical and chemical properties. Content: Substances are classified according to their physical and chemical properties. Metals are a class of elements that exhibit physical properties, such as conductivity, and chemical properties, such as producing salts when combined with nonmetals. 5.2.8.A.6 Determine whether a substance is a metal or nonmetal through student-designed investigations. Content: Substances are classified according to their physical and chemical properties. Acids are a class of compounds that exhibit common chemical properties, including a sour taste, characteristic color changes with litmus and other acid/base indicators, and the tendency to react with bases to produce a salt and water. 5.2.8.A.7 Determine the relative acidity and reactivity of common acids, such as vinegar or cream of tartar, through a variety of student-designed investigations. Content: How does conservation of mass apply to the interaction of materials in a closed system? 5.2.8.B.1Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products remain constant. Content: Chemical changes can occur when two substances, elements, or compounds react and produce one or more different substances. The physical and chemical properties of the products are different from those of the reacting substances. 5.2.8.B.2 Compare and contrast the physical properties of reactants with products after a chemical reaction, such as those that occur during photosynthesis and cellular respiration. Student Learning Targets/Objectives: should be connected to content statements. Students will be able to: e.g.: 1- Articulate predictions and imagine ways to test those predictions, using practice gained through class explorations of water quality in the school pond and water supply. 1. describe the four states of matter, the properties of each and how the states or phases change from one to another when energy is added or removed. 2. identify and understand what physical properties are and be able to identify physical and chemical properties of various objects and substances, creating their own lists. 3. understand the differences between physical and chemical changes and be able to tell the type of change from examples. 4. read the Periodic Table of Elements and be able to find basic information about the elements. 5. describe the history of the atom, parts of the atom, and the atomic theory. 6. learn to calculate the number of protons, neutrons & electrons from the periodic table. 7. understand, identify and differentiate the molecular differences between elements, compounds, mixtures and solutions and be able to identify them based on formulas and properties. 8. identify compounds and how to read their formulas. 9. Identify the four types of chemical reactions. 10. understand the law of conservation of mass and balance chemical reactions. 11. understand the properties and differences between acids and bases. Strategies/Justifications 1. Notebooking will help students clarify their own thinking, identify weaknesses and document progress. 2. Probe and lesson starter questions will help direct student thinking, introduce the topic and uncover misconceptions. 3. Use of Turning Point clickers will reinforce ideas, check for understanding and measure prior knowledge 4. Labs and activities will provide hands on inquiry based learning and allow students to work together collaboratively to solve a problem or discover an enduring understanding 5. The position project will allow students to research and present their side to a controversial issue regarding genetic engineering and present their arguments in a creative form. 6. Online resources give visual aid to the students and present information in a different way. Activities, Lessons, and Assessments Time Frame Teaching Point: There are four states of matter. Each one has unique properties with allow them to be identified. The states or phases of matter can change when energy is applied or removed. Poll students on what matter is and the two properties all matter must have (mass and volume). Diagram and discuss how changes in phase are a result of energy (usually in the form of heat) being added or removed Notes on phases of matter 1-2 hours Teaching Point: Physical properties are those that can be observed and involve the senses. Physical changes are changes in appearance only and do not change what the substance actually is. Chemical properties are those that can be tested for. Chemical changes are those that will change the substance into something new with new properties. Examples to tell a chemical change has taken place are: the addition of heat, the removal of heat, and the creation of a gas or a precipitate. 5-7 hours Focus Question: How can physical and chemical properties be used to tell apart substances that look exactly alike? Go over notes for physical and chemical properties. Have students work together to come up with examples. Through demonstration and examples, have students decide if changes are physical or chemical. Go through notes. Substance (powder) lab (2 days) will be attached. Chapter assessment on Matter Teaching Point: The periodic table of the elements can be used to identify a host of information about any element (name, symbol, atomic #, atomic #, protons, neutrons, electrons, metal or nonmetal, phase, period and family). It is arranged in a logical order, based on the atomic number and properties of the elements. Focus questions: What makes one element/atom different from another? Alien Periodic Table activity to show how periods and families work (will be attached) Color code periodic table for states of matter. Teach students how to determine the information from the table for any element. Go over history of the atom, atomic theory and what makes up the atom. Draw the structure of the atom and have students learn to calculate the number of protons, neutrons and electrons in the atom. (dittos, class notes) 8-10 days Teaching Point: Combinations of substances can be described as elements, compounds, mixtures, solutions, colloids or suspensions, depending on how the atoms are arranged. Focus Question: How are compounds different from mixtures? Notes and diagrams on combinations of substances. Use nuts, bolts and washers to demonstrate the difference between the various combinations. Activity: separating iron filings, sand and salt (attached) Use atom kits to build and name compounds Activity on naming and drawing atoms and compounds. Assessment on Atoms 6 hours Teaching Point: Chemical Reactions are the result of breaking, joining or rearranging chemical bonds. There are 4 types of chemical reactions that will illustrate the different reactions. Catalysts can assist in changing the reaction rate. Focus question: What factors control how quickly a chemical reaction occurs? Notes on chemical formulas and chemical reactions. Elephant’s toothpaste activity Milk & vinegar mini experiment (use other ingredients as well to show reaction results) Steel wool soaked in vinegar will create a rise in temperature. Flame test lab 7 hours Teaching Point: The Law of Conservation of Mass states the matter cannot be created or destroyed, it can only change forms. Reactions must follow this law, whether they are in an open or closed system. By balancing equations, the Law can be proven as well. Focus Question: Will the mass of a match before it is burned be equal to the mass after? Defend your position. Open & closed system activity Notes on law of conservation of mass Balancing chemical equations. 8 hours Teaching Point: One category of chemicals are acids and bases. They can be identified in the presence of an indicator. The pH scale is used to rate the strength of the acid or base. A neutralization reaction is acid + base salt + water. Focus question: How is a pH scale useful in a lab and how is it created? Notes on properties of acids and bases Notes on pH scale. Labe to create pH scale using various indicators. Assessment on Reactions 5 hours Resources that need to be ordered or located. Micro Burners Butane Fuel Balance Scales Assorted Glassware (beakers, graduated cylinders etc) Thermometers Stirring rods Ringstands Various chemicals Goggles Non-latex gloves Distilled water Soil testing kits Heat lamps Filter paper Funnels Electrolysis kit Technology to be integrated (tools, equipment, software, and online learning) Explorelearning.com Brainpop.com Teacherdomain