MSP Science Vocabulary – CHEMISTRY
advertisement

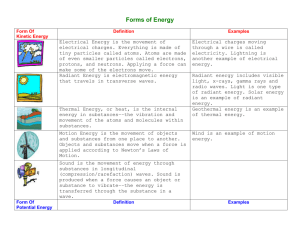
MSP Science Vocabulary – CHEMISTRY MSP Science Vocabulary – CHEMISTRY Atom – the smallest, most basic unit of matter. Everything is made up of atoms. Chemical Change – A change that occurs when one or more substances change into entirely new substances. Closed System – a system that has no input or output (ex: a closed pop bottle) Composition – What something is made out of Compound – A substance made up of atoms of two or more different elements joined by chemical bonds. Condensation – Phase change from a gas to a liquid Density – How “packed together” the atoms in a piece of matter are Element – a substance that cannot be separated or broken down into simpler substances. Listed on the Periodic Table. Evaporation – A phase change from liquid to gas Freezing – A phase change from liquid to solid Kinetic Energy – the energy of motion Mass – The measure of the amount of matter in an object Matter – anything that has mass and takes up space Melting – A phase change from solid to liquid Milliliter (mL) – 1/1000th of a Liter. Unit of liquid volume measurement Mixture – A combination of two or more substances that are not chemically combined Molecule – More than one atom together in a bond (Ex: H2O) Solubility – The ability of a substance to dissolve in another State of Matter – the physical forms of matter. Solid, liquid and gas Sublimation – Phase change where a solid goes directly into a gas Thermal (Heat) Energy – The energy of heat Volume – How much space a piece of matter takes up Atom – the smallest, most basic unit of matter. Everything is made up of atoms. Chemical Change – A change that occurs when one or more substances change into entirely new substances. Closed System – a system that has no input or output (ex: a closed pop bottle) Composition – What something is made out of Compound – A substance made up of atoms of two or more different elements joined by chemical bonds. Condensation – Phase change from a gas to a liquid Density – How “packed together” the atoms in a piece of matter are Element – a substance that cannot be separated or broken down into simpler substances. Listed on the Periodic Table. Evaporation – A phase change from liquid to gas Freezing – A phase change from liquid to solid Kinetic Energy – the energy of motion Mass – The measure of the amount of matter in an object Matter – anything that has mass and takes up space Melting – A phase change from solid to liquid Milliliter (mL) – 1/1000th of a Liter. Unit of liquid volume measurement Mixture – A combination of two or more substances that are not chemically combined Molecule – More than one atom together in a bond (Ex: H2O) Solubility – The ability of a substance to dissolve in another State of Matter – the physical forms of matter. Solid, liquid and gas Sublimation – Phase change where a solid goes directly into a gas Thermal (Heat) Energy – The energy of heat Volume – How much space a piece of matter takes up