2012 Seminar, Institute of Food Science & Biotechnology, NCHU
advertisement

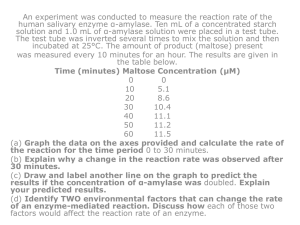
國立中興大學 103 學年度第二學期 食品暨應用生物科技學系 碩士班一年級專題討論 Title:A thermostable α-amylase from a moderately thermophilic Bacillus subtilis strain for starch processing. Author:M. Asgher, M. Javaid Asad, S.U. Rahman, R.L. Legge Impact Factor:2.576 5-Year Impact Factor:2.984 Speaker:陳柏亞 Moderator:王裕翔 Advisor:陳錦樹教授 Date: 2015/5/8(第七位) Introduction Amylases are enzymes which hydrolyse starch molecules to give diverse products including dextrins and progressively smaller polymers composed of glucose units. Amylases having approximately 25% of the enzyme market have almost completely replaced chemical hydrolysis of starch in starch processing industry. Thermostability is a desired characteristic of most of the industrial enzymes. Thermostable α-amylases are available from the mesophile Bacillus licheniformis, Bacillus sp. ANT-6 and Bacillus sp. ASMIA-2. Each application of α-amylase requires unique properties with respect to specificity, stability, temperature and pH dependence. Material and Methods Selection and isolation of bacterial strain B. subtilis JS-2004 was a moderate thermophilic bacterium. The bacterial colonies appearing on plate I were transferred to medium II. Amylase producing colonies were selected by flooding the media II plates with iodine solution. Enzyme production medium The enzyme production was carried out in the basal medium of the following composition (%): 0.1 KH2PO4, 0.25 Na2HPO4, 0.1 NaCl, 0.2 (NH4)2SO4, 0.005 MgSO4 ∙ 7H2O, 0.005 CaCl2, 0.2 tryptone, and one waste potato starch powder. Optimization of medium and culture conditions Initially, the organism was grown in the liquid medium for 24–96 h at pH 7.0 and 40˚C and then, supplemented with calcium (10 mM), yeast extract (0.5%), and glucose (0.5%) to study the effect of these nutrients on growth and enzyme production by B. subtilis JS-2004. α-Amylase assay One unit (U) is defined as the amount of enzyme which releases 1 μmol of reducing end groups per minute in 0.1 M sodium phosphate buffer (pH 7.0) with 0.5% (w/v) soluble starch as substrate at 37˚C. Effect of pH on enzyme activity and stability To determine the stability of α-amylase, the enzyme was pre-incubated in different buffers (pH 5–10) for 60 min. Effect of temperature on enzyme activity and stability The effect of temperature on amylase stability was determined by measuring the residual activity after 1 and 24 h of pre-incubation in 0.1 M sodium phosphate buffer (pH 7.0), at temperatures ranging from 40 to 100˚C. Effect of metal ions on enzyme activity Enzyme assay was performed after pre-incubation, at 60˚C (optimum) for 60 min, of the enzyme with various metal ions each at a concentration of 2 mM. Results Characterization of bacterial strain The B. subtilis JS-2004 strain was gram positive. The pH for growth was 7.0 and optimum temperature for growth was 50˚C. The strain B. subtilis JS-2004 possessed the ability to produce α-amylase and hydrolyze starch. Amylase production It was observed that maximum α-amylase production by B. subtilis JS-2004 occurred when cell population reached the peak. Enhanced bacterial growth and enzyme activity may be the result of increased availability of calcium ions. The result suggests that growth and synthesis of α-amylase by B. subtilis JS-2004 is favored by yeast extract. A decrease in cell growth and enzyme production was observed when glucose was added to the fermentation medium. There was a stimulation of enzyme synthesis with an increase in pH from 5 to 7. Enzyme synthesis occurred at temperatures between 30 and 50˚C. Characterization of crude α-amylase The α-amylase of B. subtilis JS-2004 strain was found to be active in very broad pH range. The optimum pH was found to be 8.0. Enzyme activity increased with temperature within the range of 40–70˚C. Optimum temperature of B. subtilis JS-2004 α-amylase was 70˚C. Results suggest that α-amylase did not require any ions for catalytic activity except Ca2+ and was activated (relative activity 117%) by calcium. Conclusions The B. subtilis JS-2004 strain produced high levels of thermostable α-amylase with characteristics suitable for application in starch processing and other food industries. References M. Asgher, M. Javaid Asad, S.U. Rahman, R.L. Legge. (2007). A thermostable α-amylase from a moderately thermophilic Bacillus subtilis strain for starch processing. Journal of Food Engineering, 79, 950–955. Paula Monteiro de Souza, Pérola de Oliveira e Magalhães. (2010). Application of microbial α-amylase in industry. Brazilian Journal of Microbiology, 41: 850-861.