In vivo immunosuppressive effects of intravenous
advertisement

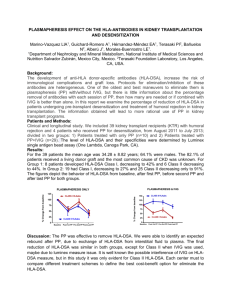
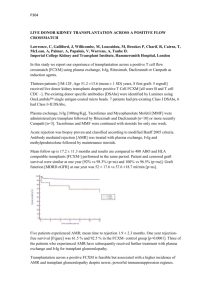
Identification of two pathways by which intravenous immunoglobulin modulates dendritic cells in humans in vivo A.S.W. Tjon1, H. Jaadar1, R. van Gent1, P. M. van Hagen2, L.J.W. van der Laan3, P.A.W. te Boekhorst4, H.J. Metselaar1, J. Kwekkeboom1 Departments of Gastroenterology and Hepatology1, Internal Medicine and Immunology2, Surgery3 and Hematology4, Erasmus MC-University Medical Centre, Rotterdam, The Netherlands. Background: High-dose intravenous Immunoglobulin (IVIg) is a safe and effective immunosuppressive therapy in autoimmune diseases. Previously, we observed that treatment with anti-HBsAg IVIg reduces the acute rejection incidence after liver transplantation, but the mode of action is not fully understood. Since myeloid dendritic cells (mDCs) play a crucial role in the initiation of cell-mediated acute rejection, we studied whether and how IVIg modulates mDCs in humans in vivo. Methods: From 28 patients treated with IVIg (indications: immunodeficiency or autoimmune disease), blood was collected before and at 1 and 7 days after IVIg infusion. In vitro, mDCs purified from blood of healthy subjects were cultured with IVIg or rIL-4 for 24hr. Surface receptors on mDC were measured by flowcytometry and plasma cytokine concentrations by ELISA. Results: Expression of the activating Fc receptor (FcR) IIa (CD32a) on circulating mDCs was reduced at 1 and 7 days after IVIg treatment (T=0→T=1: -30%, p=0.03; T=0→T=7: -22%, p=0.02), while expression of the inhibitory FcRIIb (CD32b) remained unchanged. In addition, 7 days after IVIg treatment, expression of the signalling part of the IFN-γ receptor (IFNGR2) on circulating mDC was 2.5-fold diminished (p=0.002). Hence, IVIg significantly balances mDC towards an inhibitory status. Recently it has been described that IVIg can modulate FcR expression on macrophages in mice by stimulating the IL-33 - IL-4 cytokine axis. Remarkably, we observed in our patients a rise in plasma IL33 levels after IVIg treatment (T=0→T=1: +179%, p<0.0001; T=0→T=7: +98%, p<0.0001), which was positively correlated with a rise in IL-4 plasma levels (r= 0.577, p<0.01). In vitro, rIL-4 inhibited CD32a and IFNGR2 expression on mDC, like we had observed in patients. In addition, IVIg also appeared to have direct effect on both CD32a and IFNGR2 expression on mDC (CD32a: -96%, p<0.01; IFNGR2: 57%; p=0.2 after 24 hrs culture in presence of IVIg). Functionally, IL-4- or IVIg-treated mDCs were less responsive to immune complex stimulation. Conclusion: IVIg treatment results in decreased CD32a and IFNGR2 expression on mDCs, which renders them refractory to immune complexes and IFN. This modulation may occur by stimulation of the IL-33-IL-4 cytokine axis or by a direct effect of IVIg. Hence, by modulating the most potent antigen presenting cells via two different pathways, IVIg may be a promising candidate for immunosuppression after organ transplantation. Word count Bootcongres: 2496/2500 characters (incl spaces) -1-