Sodium and potassium nitrate(III)
advertisement
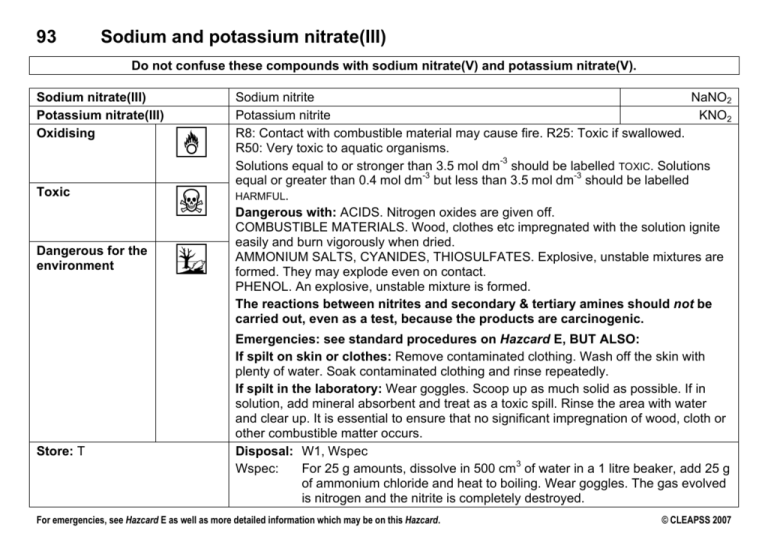
93 Sodium and potassium nitrate(III) Do not confuse these compounds with sodium nitrate(V) and potassium nitrate(V). Sodium nitrate(III) Potassium nitrate(III) Oxidising O Toxic T Dangerous for the environment N Store: T Sodium nitrite NaNO2 Potassium nitrite KNO2 R8: Contact with combustible material may cause fire. R25: Toxic if swallowed. R50: Very toxic to aquatic organisms. -3 Solutions equal to or stronger than 3.5 mol dm should be labelled TOXIC. Solutions -3 -3 equal or greater than 0.4 mol dm but less than 3.5 mol dm should be labelled HARMFUL. Dangerous with: ACIDS. Nitrogen oxides are given off. COMBUSTIBLE MATERIALS. Wood, clothes etc impregnated with the solution ignite easily and burn vigorously when dried. AMMONIUM SALTS, CYANIDES, THIOSULFATES. Explosive, unstable mixtures are formed. They may explode even on contact. PHENOL. An explosive, unstable mixture is formed. The reactions between nitrites and secondary & tertiary amines should not be carried out, even as a test, because the products are carcinogenic. Emergencies: see standard procedures on Hazcard E, BUT ALSO: If spilt on skin or clothes: Remove contaminated clothing. Wash off the skin with plenty of water. Soak contaminated clothing and rinse repeatedly. If spilt in the laboratory: Wear goggles. Scoop up as much solid as possible. If in solution, add mineral absorbent and treat as a toxic spill. Rinse the area with water and clear up. It is essential to ensure that no significant impregnation of wood, cloth or other combustible matter occurs. Disposal: W1, Wspec 3 Wspec: For 25 g amounts, dissolve in 500 cm of water in a 1 litre beaker, add 25 g of ammonium chloride and heat to boiling. Wear goggles. The gas evolved is nitrogen and the nitrite is completely destroyed. For emergencies, see Hazcard E as well as more detailed information which may be on this Hazcard. © CLEAPSS 2007 93 Sodium and potassium nitrate(III) Activity Use of solutions User Y9 Control measures Wear eye protection. Preparation of nitrogen Y9 Wear eye protection. Diazotisation of aromatic amines Y12 Wear eye protection. Do not prepare any that involve the use of old samples of naphthalenols. Preparation of nitric(III) acid (nitrous acid) Y12 Preparation of ammonium nitrite - Wear eye protection. This must be made just before it is required. A fume cupboard may be required for larger volumes of material. Do not attempt to prepare the solid salt. Model risk assessments Experimental points Use a 0.1 mol dm solution prepared by a teacher or technician. It decolourises acidified potassium manganate(VII) solution. It produces iodine from potassium iodide solution with the release of nitrogen monoxide gas. With iron(II) sulfate(VI) solution, it produces a brown colouration. 3 -3 3 Mix 2 cm of 1 mol dm ammonium chloride and 2 cm of -3 1 mol dm sodium nitrite solutions. Do not warm. Older students can use larger quantities. Some azo dyes are suspect carcinogens. Check that diazotisation is complete by testing for excess nitric(III) acid before proceeding to the next reaction step. Where possible, prepare water-soluble dyes such as methyl orange. See also Hazcard 4A. 3 -3 Add 1 g of sodium nitrite to 10 cm of ice-cold 1 mol dm sulfuric(VI) acid. On warming, nitrogen oxides (TOXIC) are produced. -3 Solutions can explode if sufficiently concentrated. For emergencies, see Hazcard E as well as more detailed information which may be on this Hazcard. © CLEAPSS 2007