Chemistry 1A Chang 6th ed
advertisement

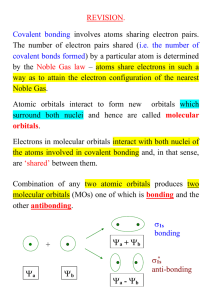
Chemistry 1A Brown & LeMay EXAM 2 STUDY GUIDE To do well on Exam 2, the student should be able to: In Chapter 6 - Electronic Structure of Atoms [Omit Photoelectric Effect pp. 230-1] Be able to calculate the wavelength () of Electromagnetic Radiation given its frequency () or its frequency given its wavelength. Be able to order the different regions in the electromagnetic spectrum according to their wavelengths or energy. Understand the concept of photons and be able to calculate their energies, using Planck's Equation (E = h). Explain how line spectra of the elements relate to the idea of quantized energy states of electrons in atoms. Use Quantum mechanics to determine what the allowed values for quantum numbers (n, l, ml, ms) are and how they relate to the number and type of orbitals. State the Heisenberg Uncertainty principle. Be able to describe the shape and explain the meaning of atomic orbitals. Write out Electron configurations of atoms (ground state and excited state) and ions using the Pauli exclusion principle and Hund's rule Identify and give the number of valence electrons in an atom. Relate Electron configuration to position of the element on the Periodic Table and determine the number of unpaired electrons in an atom; (diamagnetism and paramagnetism). Suggested Exercises: 6.9, 6.11, 6.13, 6.15, 6.17, 6.21, 6.23, 6.25, 6.31, 6.49, 6.51, 6.53, 6.55, 6.59, 6.63, 6.65, 6.66, 6.67, 6.68, 6.69, 6.70, 6.72, 6.74, 6.99. In Chapter 7 - Periodic Properties of the Elements Understand the Development of the Periodic Table and Periodic Trends in Physical and Chemical Properties. Use the Periodic Table to predict trends in Specific Physical Properties such as Atomic Radii, Ionic Radii, (Ion size in an isoelectronic series), Ionization Energy and Electron Affinity. Understand how the ionization energy changes as we remove successive electrons and recognize the jump in ionization energy that occurs when removing a core electron. Understand Electron Affinity and meaning of sign associated with its value. Suggested Exercises: 7.7, 7.11, 7.15, 7.23, 7.25, 7.27, 7.28, 7.29, 7.30, 7.31, 7.33, 7.35, 7.39, 7.41, 7.43, 7.45, 7.47, 7.49, 7.55, 7.94, 7.96. In Chapter 8 - Basic Concepts of Bonding. [Omit Formal Charge pp. 316-8] Write Lewis Dot Symbols for elements (atoms) or ions and Lewis structures for compounds. Describe in terms of electron pairs Ionic Bonding and Covalent Bonding. Understand Lattice Energy of Ionic Compounds (as a periodic property), and use the BornHaber Cycle to calculate the value of any energy term in the cycle. Use the Periodic Table to predict trends in Electronegativity. Identify whether a bond is polar, nonpolar or ionic, using Electronegativity differences as a measure of bond polarity. Explain Resonance and recognize where resonance structures are needed to describe the bonding. Understand the relationship between bond type – single or multiple covalent bonds and bond length and bond strength or enthalphy (energy). Recognize Exceptions to the Octet Rule and draw accurate Lewis structures for compounds even when the octet rule in not obeyed. Use Bond Dissociation Energy (bond enthalpies) to calculate of H of a reaction. Suggested Exercises: 8.1, 8.7, 8.8, 8.9, 8.11, 8.12, 8.13, 8.17, 8.19, 8.22, 8.27, 8.29, 8.31, 8.33, 8.35, 8.37, 8.39, 8.45, 8.46, 8.53, 8.55, 8.57, 8.59, 8.61, 8.63a, 8.65, 8.67, 8.69, 8.79, 8.91, 8.97, 8.103. To do well on Exam 2, the student should be able to: In Chapter 9- Molecular Geometry and Bonding Theories Describe the three-dimensional shapes of molecules using the VSEPR model– (Valence Shell Electron Pair Repulsion Model. (see Table 9.2 p 347 and 9.3 p 350), Determine whether a molecule is polar or nonpolar based on its geometry and the individual bond dipole moments. Identify Bonding domains (electron pairs) and nonbonding domains or lone pairs. Give the difference between Electron (Domain) Geometry and Molecular Geometry. Identify the Hybridization state of atoms in molecules. Sketch out how orbitals overlap and form sigma () bonds and/or pi () bonds Explain the concepts of bonding molecular orbitals and anti-bonding orbitals. Fill in the Molecular Orbital energy level diagram and calculate bond order. Understand the relationships between bond order, bond strength (bond dissociation energy) and bond length. Suggested Exercises: 9.7, 9.11, 9.15, 9.17, 9.19, 9.21, 9.22, 9.25, 9.29, 9.31, 9.33, 9.35, 9.37, 9.39, 9.43, 9.45, 9.47, 9.49, 9.51, 9.53, 9.59, 9.61, 9.62, 9.63, 9.65, 9.67, 9.68, 9.69, 9.71, 9.76, 9.81, 9.87, 9.97. You may omit “A closer look” sections on pages 214, 225, 265, 318, 367 and 373-4. Laboratory Experiments: No questions on the spectro lab (expt 6) or its calculations. Problems on Molecular Geometry like those in Experiment 7 and VSPER Exercise are on the exam.