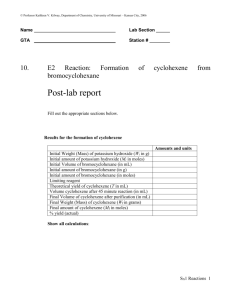
dehydration
advertisement

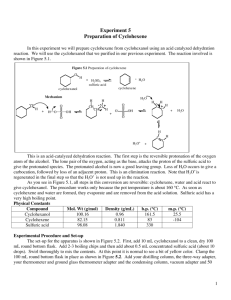
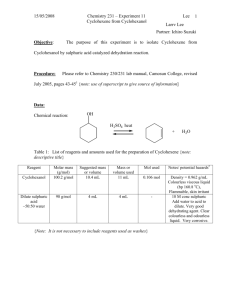
Experiment 2 Preparation of cyclohexene Object (a) To prepare cyclohexene by dehydrating cyclohexanol using concentrated phosphoric (V) acid (b) To introduce the method of purification for an organic liquid Theory Cyclohexanol can be dehydrated to form cyclohexene by using a dehydrating agent. Concentrated phosphoric (V) acid is better than concentrated sulphuric acid in this reaction. Apparatus Quick-fit apparatus, water bath, hot plate, separating funnel Chemical cyclohexanol, conc. phosphoric (V) acid, saturated sodium chloride Solution, anhydrous calciuml chloride Procedure Dehydration: Place 40 g of cyclohexanol in a pear-shaped flask, then add slowly with swirling 3 cm3 of concentrated sulphuric acid and mix the contents of the flask thoroughly. Fit the flask with a fractionation column which is attached to a condenser fitted with an adapter that passes well into a 100 cm3 conical flask surrounded by an ice-water bath. Heat the flask gently over a wire gauze with a small flame, so that the cyclohexene and water distill through the column. The temperature observed at the top of the column is below the boiling point of cyclohexene (why?), unless distillation is excessively fast. out Distillation is continued until no more cyclohexene distills and a dark condenser residue is left in the flask. This usually requires 15-20 minutes. Purification: Transfer the distillate to separating funnel, add about 5 cm3 of 10% sodium carbonate solution to neutralize traces of acid, than shake the mixture carefully. (Caution: Remember to release pressure from generation of carbon dioxide). After the layers have separated, discard the lower water layer carefully, then run the cyclohexene layer into a small, dry conical fractionating column flask. Add about 3-4 g of anhydrous calcium chloride to the flask, cork the flask tightly to prevent loss of the volatile product, and than allow the mixture to stand for about an hour with occasional swirling. Decant the dried product through a gravity funnel containing a small filter paper into a receiver small, dry distillation flask, than distill. Collect the product over a range of about five degree around 83C (the reported b.p. of cyclohexene). Test: 1. Add the product dropwise into 1cm3 of bromine solution in tetrachloromethane. Note the changes. Record the number of drops required. 2. Add the product dropwise into 1cm3 of acidified potassium permanganate solution. Note the results, and record the number of drops added. Notes 1. Alternative methods of alkene synthesis include elimination of hydrogen halide (equation 3) and cracking of an ester (equation 4). These two methods have the advantage that the carbonium ion is not an intermediate, so rearrangement does not occur. In the direct dehydration with acid, where the carbonium ion is normally expected as a reaction intermediate, rearrangement to isomeric carbonium ion occurs rapidly in instances where isomeric ions are structurally possible. In the case of cyclohexene preparation, rearrangement of the carbonium ion can lead to the same structure, so a single alkene is conveniently obtained by this simple method of dehydration. 2. Side reactions in this preparation include acid-catalysed polymerization of alkeneoxidation by hot sulphuric acid, formation of dicyclohexyl ether, and equilibration with cyclohexyl hyrdogensulphate (equation 5). As ether formation is quite slow below 125C, so this is a minor side reaction in the formation of an alkene from a secondary alcohol. 3. From equilibrium, removal of water is desirable in order to displace the equilibrium forward (although the equilibrium in equation 5 is also displaced forward). Finally, the removal of cyclohexene from the reaction mixture as rapidly as formed is desirable for two reasons: the equilibrium is shifted forward, and the acid-catalysed polymerization of the alkene is minimized. 4. Removal of both cyclohexene and water from the reaction mixture is accomplished by carrying out the reaction under a fractionating column and adjusting the heat so that there is slow fractional steam distillation. This delivers the more volatile cyclohexene as the principal component of the organic phase in the distillate and returns essentially all the much higher boiling cyclohexanol to the flask for further reaction with hot acid. Removal of the volatile constituents by steam distillation also allows a Preparation of cyclohexene / page 1 high enough temperature in the flask to give a rapid rate of dehydration. Thus, running the reaction under the fractionating column allows ideal reaction conditions. Questions 1. Concentrated phosphoric (V) acid is preferred to concentrated sulphuric acid as a dehydrating agent for alkanols because it gives a higher yield of alkene. Why does concentrated sulphuric acid give a lower yield of alkene? 2. Name two impurities present in the cyclohexene before purification. 3. What impurities are removed from the impure cyclohexene when it is shaken with sodium chloride solution? 4. Why is the cyclohexene mixed with anhydrous calcium chloride? 5. What are the three key stages in purifying an organic liquid? 6. What mass of cyclohexene did you obtain? 7. Use the equation for the dehydration of cyclohexanol to calculate the theoretical yield of cyclohexene. 8. What is your percentage yield of cyclohexene? 9. Suggest four products formed when the cyclohexene burns. 10. Write an equation for the complete combustion of cyclohexene in excess oxygen. 11. Describe what happens, write an equation for the reaction between cyclohexene and bromine, and name the product. 12. Describe what happens, write an equation for the reaction between cyclohexene and concentrated sulphuric acid. 13. Many of the reactions of alkenes involve addition. What do you understand by the term of addition reaction? Preparation of cyclohexene / page 2