Electronic Supplementary Material Electrochemical immunosensor
advertisement

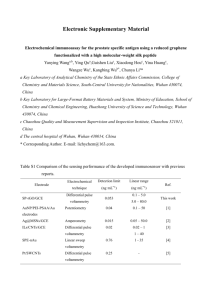
Electronic Supplementary Material Electrochemical immunosensor for the prostate specific antigen detection based on carbon nanotube and gold nanoparticle amplification strategy Jing Yang, Wei Wen, Xiuhua Zhang, Shengfu Wang* Hubei Collaborative Innovation Center for Advanced Organic Chemical Materials, Ministry of Education Key Laboratory for the Synthesis and Application of Organic Functional Molecules & College of Chemistry and Chemical Engineering, Hubei University, Wuhan 430062, PR China ________________________ *Corresponding author, Fax: +86-27-88663043; Telephone: +86-27-50865309; E-mail address: wangsf@hubu.edu.cn (S. Wang) Characterization of gold nanoparticles The gold nanoparticle was characterization by UV–vis spectrum and Transmission Electron Microscopy (TEM). As shown in Fig. S1, an absorption peak at 520 nm was observed in the UV–vis spectrum of AuNPs in water and an average diameter of approximately 13 nm as measured by TEM. 1 Fig. S1 UV-vis characterization of the gold nanoparticle, insert is the TEM image of AuNPs. Grafting 1, 7-diaminoheptane on glassy carbon electrode The electrochemical modification of the GCE surface with a monolayer of 1, 7-diaminoheptane was carried out by applying a potential to the electrode between 0.2 V and 1.6 V for three cycles at a scan rate of 20 mV﹒s−1 in an absolute ethanol solution containing 2 mM 1, 7-diaminoheptane and 0.1 M NaClO4. A broad and irreversible oxidation peak was observed at 1.30 V, shown in Fig. S2. 25 Current / mA 20 1 15 2 10 3 5 0 -5 0.0 0.3 0.6 0.9 1.2 1.5 1.8 Potenvial / V Fig. S2 Cyclic voltammograms of a freshly polished GCE upon scanning in 2 mM DAH and 0.1 M NaClO4 ethanol solution. (1) First, (2) second and (3) third cycles. 2 Optimization of experimental conditions In order to provide the immunosenor with a better performance, the volume ratio of Ab2 to Fc, the volume of Ab2 when prepare the Ab2-Fc-AuNPs conjugates, and the incubation time of Ab1 and Ab2-Fc-AuNPs with PSA were investigated. The process for preparation of the Ab2-Fc-AuNPs conjugates was optimized to achieve the best DPV performance. The volume ratio of Ab2 (0.1 mg﹒mL−1) to Fc (5mM) was studied from 4:1 to 2:3 as shown in Fig.S3A. We found that a volume of 4:1 gave the highest DPV signal response, which indicated the linking efficiency of Ab2 to Fc at the certain ratio is relatively the highest. When the volume ratio is higher than 4:1, less Ab2 molecules were linked to the AuNPs. In this regard, less amount of Ab2-Fc-AuNPs conjugates combined with the PSA resulted in a decrease in the electrochemical signal. So the volume ratio of 4:1 was chosen for the subsequent assays. Under the optimized volume ratio of Ab2 to Fc, the dosage of the Ab2 also affected the efficiency of the Ab2-Fc-AuNPs conjugates binds to PSA, which would influence the DPV signal. As shown in Fig. S3B, with an increase in the amount of Ab2, the current value gradually increased and then became nearly balanced when the volume of Ab2 was 250 μL. Therefore, the optimum dosage of the Ab2 was chosen as 250 μL. Fig. S3 (A) Optimization of the experimental condition for DPV response to the different volume ratio of AuNPs modified Ab2 (0.1 mg﹒mL−1) and Fc (5mM). (B) Optimization of the experimental condition for DPV response to different volume of Ab2. 3 The effect of incubation time between Ab1 and PSA (Fig.S4A), Ab2-Fc-AuNPs and PSA (Fig.S4B) of the immunosensor were examined from 15 to 120 min, respectively. It is found that the peak current response of the immunosensor to 100 ng﹒mL−1 PSA increases with increasing incubation time, and reached a platform till 75 min (Fig.S4A) and 90 min (Fig.S4B). This result suggests that the immunoreaction of the antibodies and PSA have entirely completed in certain time. Thus, 75 min and 90 min were adopted in this work for the incubation time of Ab1 and PSA, Ab2-Fc-AuNPs and PSA, respectively. Fig. S4 DPV peak currents plotted against incubation time of the Ab1 modified electrode (A) and Ab2-Fc-AuNPs conjugates (B) with PSA (100 ng﹒mL−1) solution at 37 °C. Controlled experiments In order to confirm the amplification strategy, we compare the results of the controlled experiments without multi-walled carbon nanotubes (MWNTs) to the immunosensor shown in Fig. S5. 4 Fig. S5 (A) DPV responses of the immunosensor without MWNTs incubated with different concentrations of PSA (From a → i: 0, 1, 10, 20, 30, 40, 60, 80, 100 ng﹒mL−1). (B) Linear relationship between the current response and PSA concentration. The error bars indicated the standard deviation of three measurements. 5