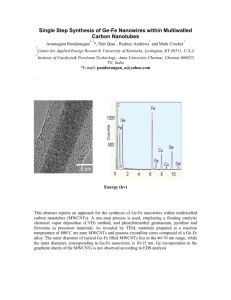
Electronic Supplementary Material Sensitive electrochemical
advertisement

Electronic Supplementary Material Sensitive electrochemical determination of α-fetoprotein using a glassy carbon electrode modified with in-situ grown gold nanoparticles, graphene oxide and MWCNTs acting as signal amplifiers Yan-Sha Gao,a,b Xiao-Fei Zhu,a Tao-Tao Yang,a Jing-Kun Xu,a* Li-Min Lu,b* Kai-Xin Zhang a a Jiangxi Key Laboratory of Organic Chemistry, Jiangxi Science and Technology Normal University, Nanchang 330013, PR China b College of Science, Jiangxi Agricultural University, Nanchang 330045, PR China Characterization of UV–vis spectrum Fig. S1 shows the UV–vis spectrum of MWCNTs (a), GO (b) and AuNPs/GO-MWCNTs (c). As shown, the absorption peak at about 270 nm is attributed to the characteristic absorption of MWCNTs [1]. While GO exhibited two characteristic peaks, a maximum at 230 nm, which corresponds to π–π* transitions of aromatic C–C bonds, and a shoulder at 300 nm, which is attributed to n→π* transitions of C=O bonds (curve b) [2]. In the UV–vis spectrum of AuNPs/GO-MWCNTs (c), besides the same absorption peak derived from GO centered at 300 nm, the absorption peak centered at 230 nm was shifted to higher wavelength (253 nm), which was indicative of strong π–π interaction between GO and MWCNTs. A peak at 523 nm was also observed (curve c), which was attributed to the formation of AuNPs [3]. Fig. S1. UV–vis absorption spectra of the MWCNTs (a), GO (b), AuNPs/GO-MWCNTs (c). Optimization of the method The goal of this study was to control the optimal experimental conditions to obtain the excellent performance of the proposed immunosensors for α-FP detection. The factors influencing the performance of the immunosensor included the concentration of HAuCl4 in the dispersion of GO, the buffer pH, the incubation temperature, and the incubation time. Fig. S2. Effect of the concentration of HAuCl4 on the response of the immunosensor to 100 ng mL-1 α-FP. The concentrations of GO was 0.5 mg mL-1. The effect of the concentration of HAuCl4 in the dispersion of GO was optimized. As shown in Fig. S2, the current responses of the immunosensor increased with the increment of HAuCl4 concentration, and then reached the highest at 5 mM. The current signal gradually decreased at higher concentration. Thus, 5 mM HAuCl4 was adopted in the following experiments. The effect of pH on the detection solution on the immunosensor behavior was investigated over a pH range from 5.5 to 8.0 with 100 ng mL-1 α-FP. As shown in Fig. S3a, the current responses increased from pH 5.5 to 7.4 to reach the maximum value and decreased from pH 7.4 to 8.0. Hence, pH 7.4 was chosen as the optimum pH of the detection solution throughout this study to obtain a high sensitivity. Temperature was an important factor for the activity of the antibody and antigen. The effect of temperature on the immunosensor response was also studied in the temperature range from 5 to 45 °C. As shown in Fig. S3b, maximum response was achieved at a temperature around 35 °C. However, temperature above 40 °C might cause irreversible denaturation of α-FP and anti-α-FP. As is well known, long-time use in high temperature may damage the modifier and affect the lifetime of the immunosensor. Taking into account the activity, lifetime, and response characteristics of biomolecules, we chose the normal room temperature of 25 °C as the measure temperature in our study. The immunosensor was incubated in a constant concentration of α-FP for different times. ∆ I rapidly increased within the first 40 min and then tended to level off. Therefore, 40 min was chosen as the optimal incubation time (shown in Fig. S3c). Fig. S3. Influence of the pH of the PBS (a), incubation temperature (b) and incubation time (c) on the current responses of the developed immunosensor. Fig. S4. (A) Plot of Q-t curves of GO-MWCNTs/GCE (a), AuNPs/GO-MWCNTs/GCE (b), in 1 mM K3[Fe(CN)6] containing 0.1 M KCl. The inset shows the Plot of Q-t1/2 curves on GO-MWCNTs/GCE (a’), AuNPs/GO-MWCNTs/GCE (b’) (B) Cyclic voltammograms of the GO-MWCNTs/GCE (a), AuNPs/GO-MWCNTs/GCE (b) in pH 7.4 phosphate buffer containing 0.1 M KCl and 5.0 mM Fe(CN)63-/4- at the scan rate of 50 mV-1. References: [1] Tang C, Zhou T, Yang J (2011) Wet-grinding assisted ultrasonic dispersion of pristine multi-walled carbon nanotubes (MWCNTs) in chitosan solution. Colloids Surf., B 86: 189-197. [2] Aboutalebi SH, Chidembo AT, Salari M, Konstantinov K, Wexler D, Liu HK, Dou SX (2011) Comparison of GO, GO/MWCNTs composite and MWCNTs as potential electrode materials for supercapacitors. Energy Environ. Sci 4: 1855-1865. [3] Zhang NN, Qiu HX, Liu Y, Wang W, Li Y, Wang XD (2011) Fabrication of gold nanoparticle/graphene oxide nanocomposites and their excellent catalytic performance. J Mater Chem 21:11080-11083.