Supporting Information - Springer Static Content Server
advertisement
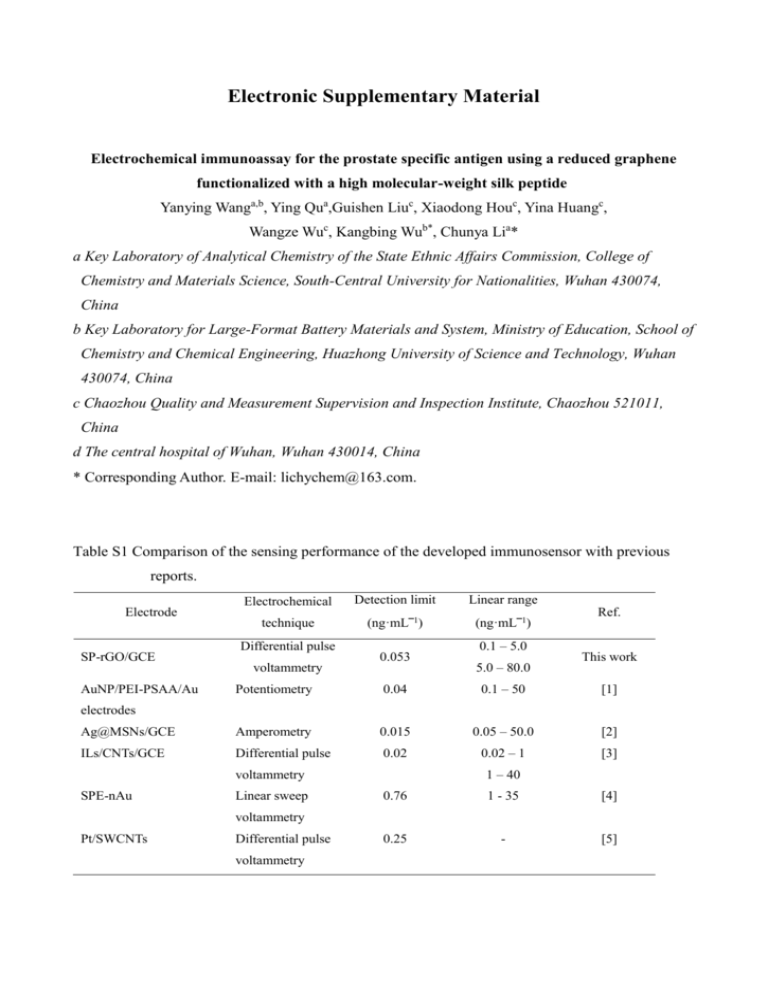
Electronic Supplementary Material Electrochemical immunoassay for the prostate specific antigen using a reduced graphene functionalized with a high molecular-weight silk peptide Yanying Wanga,b, Ying Qua,Guishen Liuc, Xiaodong Houc, Yina Huangc, Wangze Wuc, Kangbing Wub*, Chunya Lia* a Key Laboratory of Analytical Chemistry of the State Ethnic Affairs Commission, College of Chemistry and Materials Science, South-Central University for Nationalities, Wuhan 430074, China b Key Laboratory for Large-Format Battery Materials and System, Ministry of Education, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, Wuhan 430074, China c Chaozhou Quality and Measurement Supervision and Inspection Institute, Chaozhou 521011, China d The central hospital of Wuhan, Wuhan 430014, China * Corresponding Author. E-mail: lichychem@163.com. Table S1 Comparison of the sensing performance of the developed immunosensor with previous reports. Electrode SP-rGO/GCE Electrochemical Detection limit Linear range technique (ng·mL‾1) (ng·mL‾1) Differential pulse voltammetry 0.053 0.1 – 5.0 5.0 – 80.0 Ref. This work Potentiometry 0.04 0.1 – 50 [1] Ag@MSNs/GCE Amperometry 0.015 0.05 – 50.0 [2] ILs/CNTs/GCE Differential pulse 0.02 0.02 – 1 [3] AuNP/PEI-PSAA/Au electrodes 1 – 40 voltammetry SPE-nAu Linear sweep 0.76 1 - 35 [4] 0.25 - [5] voltammetry Pt/SWCNTs Differential pulse voltammetry Fig. S 1 Morphologic characterization of the SP-rGO nanocomposites (a) and the rGO nanosheets (b) with transmission electron microscopy. From the comparison of TEM images of SP-rGO and rGO we can obviously see that modification has no significant on the morphology of nanosheets. Fig. S 2 X-ray diffraction characterization of the GO (a), the rGO (b) and the SP-rGO (c). Disappearing of the sharp peak located around 10° indicates that the reduction of the GO to the rGO. Fig.S3 X-ray photoelectron spectroscopy characterizations of the SP-rGO (a) and the r-GO (b). Existence of N1s in the SP-rGO nanocomposites means the conjugation of silk peptides onto the rGO nanosheets. Fig. S 4 FTIR spectra of the GO (a), the SP (b) and the SP-rGO (c). Some characteristic peaks of the SP and the GO were observed in the SP-rGO, especially vC-N at 1385.69 cm-1, indicate the conjugation of the SP and the rGO. Fig. S 5 Roman spectra of the GO (a) and the SP-rGO (b). The Raman spectra of graphene oxide nanosheets (a) and high molecular-weight silk peptide/reduced graphene oxide nanosheets (b) were shown in Fig. S 5. G-band located at 1591 cm-1 is a result of in-plane vibrations of SP2 bonded carbon atoms. The peak appeared at 1351 cm-1 is known as the D-band which is due to out of plane vibrations attributed to the presence of structural defects. Comparing the ratio of the intensitiy of D/G, it is clearly to see that the ID/IG of the GO nanosheets is obviously higher than that obtained from SP-rGO nanosheets. The result demonstrates that the reduction of the graphene oxide nanosheets to be the reduced graphene oxide nanosheets have been successfully achieved. Fig. S 6 Effects of pH values (A), incubation temperature (B) and incubation time (C) on the peak current response of the immunosensor towards 50.0 ng·mL-1 PSA. Based on the current response of the immunosensor towards 50.0 ng·mL-1 PSA, the influences of pH values (A), incubation temperature (B) and incubation time (C) were investigated and shown in Fig. S 6. The peak current difference (∆I) before and after the immunosensor being incubated into PSA solution was used as a criterion to evaluate their effects. pH value of the buffer solution greatly affected the immunosensor for selective recognition of PSA. From Fig. S 6 (A), it can be seen that the current response sharply increase with the pH values varied from 4.0 to 6.0. With further increasing of pH values, the current response decreased reversely. A maximum current response was obtained at pH value of 6.0, therefore, it was chosen for the further experiments to obtain high sensitivity. Incubation temperature can have strong effects on the bioactivity of anti-PSA and may influence the specific binding between the immunosensor and PSA, thus further affect the current response of the immunosensor. As shown in Fig. S 6 (B), it was found that the ΔI values of 50.0 ng·mL–1 PSA increases with the variation of incubation temperature from 20 ºC to 35 ºC. However, a slight decrease was observed when the incubation temperature was varied from 35 ºC to 45 ºC, meaning that a high temperature may cause the ineffective binding of PSA. Therefore, 35 ºC was selected as the optimized incubation temperature for the selective recognition of PSA by the immunosensor. The incubation time for the binding reaction between anti-PSA and PSA is also critically important for the immunoassay. Fig. S 6 (C) shows the dependence of the current response on the incubation time. The current response of the immunosensor almost reaches the maximum value after being incubated into 50.0 ng·mL-1 of PSA for 30 min. The result indicates that the binding reaction can be finished when the incubation time is 30 min. In order to reduce the analytical time and obtain relatively high sensitivity, therefore, 30 min was employed as the incubation time in the following experiments. Fig. S 7 Current response of the immunosensor to (a) 10 ng·mL-1 PSA and (b) 10 ng·mL-1 PSA in the presence of 100.0 ng·mL-1 AFP, 100.0 ng·mL-1 human IgG, 1.0 μg·mL-1 BSA, 1.0 μg·mL-1 Vc, 1.0 μg·mL-1 L-Cysteine, and 1.0 μg·mL-1 L-Lysine. Error bars represent standard deviation (n =3). References [1] B. Zhang, B. Q. Liu, G. N.Chen, D. P.Tang, Biosens. Bioelectron. 53 (2014) 465. [2] H. Wang, Y. Zhang, H. Q. Yu, D. Wu, H. M. Ma, H. Li, B. Du, Q. Wei, Anal. Biochem. 434 (2013) 123. [3] A. Salimi, B.Kavosi, F. Fathi, R. Hallaj, Biosens. Bioelectron. 42 (2013) 439. [4] V. Escamilla-Gómez, D. Hernández-Santos, M. B. González-García, J. M. Pingarrón-Carrazón, A. Costa-García, Biosens. Bioelectron. 24 (2009) 2678. [5] J. Okuno, K. Maehashi, K. Kerman, Y. Takamura, K. Matsumoto, E. Tamiya, Biosens. Bioelectron. 22 (2007) 2377.










