Determination of the Formula of a Hydrate
advertisement

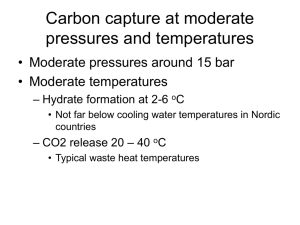
Determination of the Formula of a Hydrate Many compounds are formed as a result of reactions that occur in water solutions. These compounds appear to be dry, but when they are heated, large amounts of water are released. The water molecules are part of the crystalline structure and are weakly bonded to the ions or molecules that make up the compound. Such compounds are known as hydrates, meaning that they contain water. The solid that remains when the water is removed is referred to as the anhydrous salt, or anhydrate. Usually the amount of water present in a hydrate is in a whole number mole ratio to the moles of anhydrate. An example of a hydrate is magnesium sulfate. Its formula is MgSO4 ●7 H2O, indicating that seven moles of water are combined with one mole of magnesium sulfate in the crystalline form. The dot does not mean time seven water molecules but plus seven molecules of water. The molecular formula of the hydrate is the mass of MgSO4 plus the mass of 7 H2O molecules. In this investigation you will be given an unknown hydrate and asked to determine the formula and the percent of water in the compound. The actual formula of the hydrate will then be given in order to determine the percent error of the experiment. Hypothesis: Calculate the expected yield of water from your hydrate. Ex: MgSO4 * 7H2O Percent of H2O = mass of H2O = 7(18.02 g/mol) x 100 = 45% H2O Mass of MgsO4* 7H2O 278.69 g/mol Expected yield = 2.00 grams of hydrate x .45 = 0.90 grams of H2O Procedure: 1. Set up the crucible and clay triangle and heat the crucible with its cover, slightly askew, in the hottest part of the flame for 3 minutes. 2. Remove the crucible using the crucible tongs and place on the counter to cool for 2 minutes. Never touch the crucible with your hands after heating it. 3. Place the crucible on the digital balance and measure out approximately 2.00 grams of the unknown hydrate. Record the number of your hydrate and its mass. 4. Heat the crucible and sample for five minutes with the cover slightly askew. 5. Remove the crucible and place on the counter with the cover completely over the sample and cool for 2 minutes. 6. Mass the crucible, cover and sample and record. 7. Reheat the crucible and sample for five minutes and cool for two and remass. If the mass is within 0.02 grams of the last mass then you are finished. If not you must reheat until the mass does not change by more than 0.02 grams. Record the final mass of the sample. 8. Dispose of the sample in the trash and clean your crucible and work area. Data: Record all your data in a table. Calculations: 1. Calculate the percent of water in your hydrate from your lab data. 3. Calculate the actual yield of water from your hydrate. 2. Determine your percent yield. Percent yield = actual yield x 100% Expected yield Analysis: 1. Define hydrate and anhydrate. 2. Why do we heat the crucible before adding the hydrate? 3. Why do we cover the crucible while cooling but leave it askew while heating? 4. How could the weather affect the results of this experiment? 5. Why do we heat the sample until the mass doesn’t change anymore? Conclusion: Restate the purpose of the experiment and give your hypothesis and the answer to the question. Discuss your percent error and give some reasons for the error, other than mistakes you may have made. How could you change this experiment to reduce your percent error?