Carbon capture using gas hydrate technology
advertisement

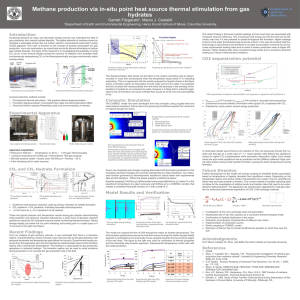
Carbon capture at moderate pressures and temperatures • Moderate pressures around 15 bar • Moderate temperatures – Hydrate formation at 2-6 oC • Not far below cooling water temperatures in Nordic countries – CO2 release 20 – 40 oC • Typical waste heat temperatures Main concept overview 99% CO2 1% N2 Flue gas 15% CO2 85% N2 A closed process involving cooling, heating, compression and decompression stages to form and melt CO2 hydrates Hydrate promoter (formation pressure) Seed particles (reaction kinetics) Electric power Cooling water Waste heat N2 Traces of CO2 The separation is possible since CO2 forms hydrates more easily than N2. The problem with hydrate processes • There is usually a long induction time before hydrate production start – Seed must form and grow to a certain size before detectable gas absorption is observed – This takes a long time – hours and days in pure systems • The hydrate forms first where the gas concentration is high – Thus a droplet gets a hydrate crust around a wet inside • This hinders the transport of gas into the water phase • And heat away from the reaction centre • Speeding up the process – the IFE contribution and a possible breakthrough – Using heterogeneous seed particle to speed up hydrate formation – induction time reduced by a factor of 200 Comparing to chilled ammonia process • From literature chilled ammonia process consumes energy in the range: – 470 – 550 kWh/ton CO2 • A first rough estimate for a hydrate process: – 220 – 330 kW/ton CO2 (0.8 – 1.2 GJ)