Worksheet
advertisement

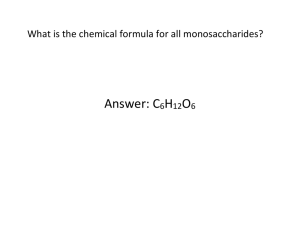
The Structure and Function of Macromolecules Carbohydrates, Lipids, Proteins (and Nucleic Acids) Name Per Directions: Use the reading, “Unit One: The Chemistry of Life” (Chapter Five: The Structure and Function of Macromolecules) online, pages 1-5, to complete the following questions. The reading is on a pdf linked on the class calendar. 1. List the four main classes of macromolecules. (Note: Nucleic Acids are not considered nutrients found in food). 2. Polymers (“many” “parts”) are made up of what smaller unit? How are these units linked to form these polymers? 3. Explain what hydrolysis is and draw what it may look like. Use boxes or small diagrams for now to represent the monomers. Carbohydrates 4. What is the general molecular formula for monosaccharides? 5. What is the range of numbers of carbon that monosaccharides usually have? 6. Three-carbon monosaccharides are called Draw what this may look like in the space below. Five-carbon monosaccharides are called Draw what this may look like in the space below. Six-carbon monosaccharides are called Draw what this may look like in the space below. 3-carbon monosaccharides 5-carbon monosaccharides 6-carbon monosaccharides 7. Glucose can be drawn in a linear skeleton. Why is this not an accurate representation of this molecule? 8. How is a glycosidic linkage created? (i.e., what is the reaction that allows a glycosidic linkage?) What molecule is lost during this reaction? 9. Write the monomers that make up the following disaccharides: Maltose Sucrose Lactose & & & 10. List the four types of polysaccharides mentioned and generally their function/purpose. 11. Which of the following terms includes all of the others in the list? Explain below how you know. a. b. c. d. e. Monosaccharide Disaccharide Starch Carbohydrate Polysaccharide 12. The molecular formula for glucose is C6H12O6. What would be the molecular formula for a polymer made by linking ten glucose molecules together by condensation reactions? Explain your answer. a. C60H120 O60 b. C6H12 O6 c. C60 H102O51 d. C60 H100 O50 e. C60 H111 O51 Lipids 1. Why are fats hydrophobic? Explain in terms of the polarity of molecules. 2. Where could you find saturated fats in nature? Where could you find unsaturated fats in nature? 3. Phospholipids are different from triglycerides in what way? Where can you find phospholipids in the human body? 4. Think about the structure of fatty acids. What do they all have in common? 5. What do double bonds do to fatty acids? 6. If you had to make a rule to spot fat molecules among different kinds of molecules, what would it be? Why? 7. Draw a fatty acid below. Draw a glycerol molecule below. 8. Explain the relationship between fats, fatty acids, and glycerol. Proteins 1. List 6 functions of proteins in the cell. 2. How many amino acids exist in nature? 3. Observe the model of an amino acid on pg. 4 (top right). What is the molecular formula for the amine group? 4. What (that is, what part of the amino acid molecule) determines how a particular amino acid will behave? 5. In carbohydrates, covalent bonds called glycosidic linkages bond two monosaccharides together. What is the protein counterpart for a glycosidic linkage? 6. Give another name for a protein polymer: 7. What is the difference between a polypeptide and a protein? 8. Explain generally how: a. primary structure of a protein is determined b. secondary structure of a protein is determined c. tertiary structure of a protein is determined d. what a quaternary structure is composed of 9. List three conditions in a protein’s environment that can impact its ability to function properly or in more extreme cases, cause denaturation.