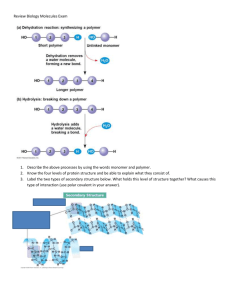
SBI 4U Name: More About Carbohydrates Date: Monosaccharides
advertisement
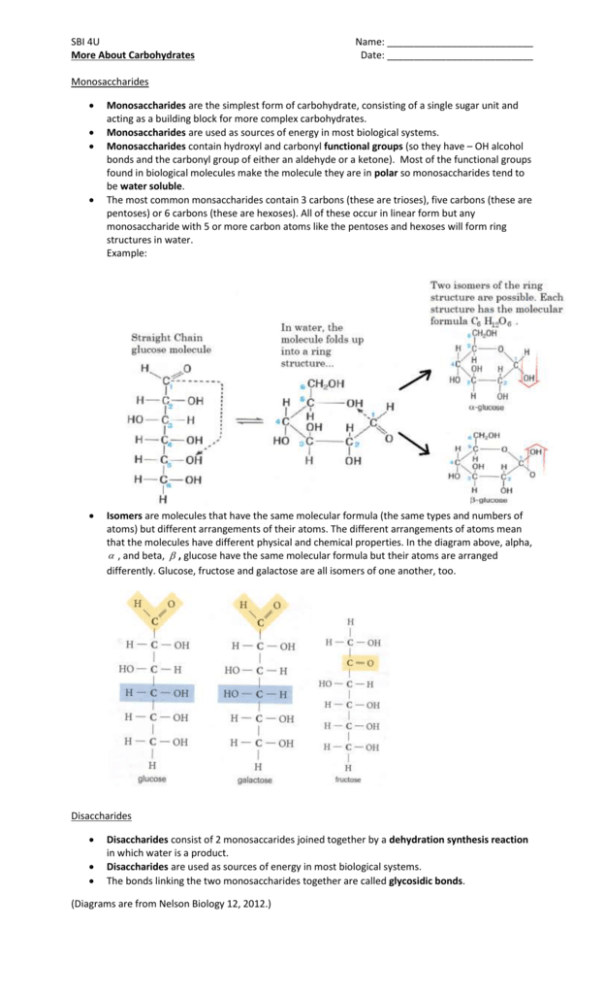
SBI 4U More About Carbohydrates Name: ___________________________ Date: ___________________________ Monosaccharides Monosaccharides are the simplest form of carbohydrate, consisting of a single sugar unit and acting as a building block for more complex carbohydrates. Monosaccharides are used as sources of energy in most biological systems. Monosaccharides contain hydroxyl and carbonyl functional groups (so they have – OH alcohol bonds and the carbonyl group of either an aldehyde or a ketone). Most of the functional groups found in biological molecules make the molecule they are in polar so monosaccharides tend to be water soluble. The most common monsaccharides contain 3 carbons (these are trioses), five carbons (these are pentoses) or 6 carbons (these are hexoses). All of these occur in linear form but any monosaccharide with 5 or more carbon atoms like the pentoses and hexoses will form ring structures in water. Example: Isomers are molecules that have the same molecular formula (the same types and numbers of atoms) but different arrangements of their atoms. The different arrangements of atoms mean that the molecules have different physical and chemical properties. In the diagram above, alpha, , and beta, , glucose have the same molecular formula but their atoms are arranged differently. Glucose, fructose and galactose are all isomers of one another, too. Disaccharides Disaccharides consist of 2 monosaccarides joined together by a dehydration synthesis reaction in which water is a product. Disaccharides are used as sources of energy in most biological systems. The bonds linking the two monosaccharides together are called glycosidic bonds. (Diagrams are from Nelson Biology 12, 2012.) Examples: A dehydration synthesis reaction between two molecules of the monosaccharide glucose result in a bond between carbon 1 of the molecule on the left and carbon 4 of the molecule on the right. This is called a 1 4 glycosidic linkage. This 1 4 linkage is an - linkage because the OH group attached to carbon number 1 is oriented downward. Water is a by-product of all dehydration synthesis reactions. The resulting disaccharide formed when 2 glucose molecules undergo a dehydration synthesis reaction to form a 1 4 -glycosidic linkage is called maltose. Maltose is important in beer making. It is also used as a food source. To the left is a 1 5 glycosidic bond between one molecule of glucose and one molecule of fructose to make the disaccharide, sucrose, or table sugar. To the left is a 1 4 glycosidic linkage between one molecule of galactose and one molecule of glucose to form one molecule of the disaccharide lactose, found in milk. Complex Carbohydrates: Polysaccharides A complex carbohydrate is formed when hundreds to thousands of monosaccharides link together in a chain. There are two basic types of complex carbohydrates: a) storage carbohydrates like starch and glycogen which store monosaccarides in plants and animals respectively, until they are needed as fuels to release energy. b) structural carbohydrates like the cellulose in plant cell walls that makes them hard and protective and the chitin that forms the hard shells or exoskeletons of insects and crustaceans. A polysaccharide molecule is a chain of monsaccharides with many subunits joined together by glycosidic linkages. These are macromolecules – very large molecules. The dehydration synthesis reactions that form the glycosidic linkages between the identical or non-identical monosaccharides (also referred to as “units” or “monomers”) are examples of polymerization reactions, reactions that link smaller units to form much larger units or polymers. Figure 11 page 32 compares the glycosidic linkages in cellulose and in starch. Humans can break down the linkages in starch and in glycogen but do not contain the digestive enzymes required to break down the linkages in cellulose. Instead, the cellulose from the cell walls of plant material acts as indigestible fibre in the human digestive tract. Other organisms like sheep, cows and rabbits contain bacteria and protists in their digestive tracts that produce the enzymes required to break down the cellulose in grass and plants so that its monomers can be used as a source of energy. Note that polysaccharides are often polar molecules and are hydrophilic. They will not dissolve in water, though, because they are too large. The cellulose molecules that paper towels are made from make them absorbent to water but do not let them dissolve away in it.