Lesson - Nstacommunities.org
advertisement

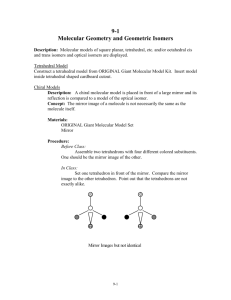
Introduction to Enantiomers and Handedness Subject Area: Chemistry Grade Level: High School Chemistry Lesson Title: Introduction to Enantiomers and Handedness National Science Education Standards: Science as Inquiry: 9-12 Physical Science Standards: Structure of atoms: 9-12 Structure and properties of matter: 9-12 Chemical reactions: 9-12 Suggested Prior Knowledge: Isomers Purpose: To give students an understanding of enantiomers and chirality and their influences on chemical reactivity. Key Vocabulary: stereoisomers - isomers that have the same connections among the atoms but have different arrangement of the atoms in space geometric or cis-trans isomers or diastereoisomers - non-mirror-image stereoisomers enantiomer - stereoisomers that are non-identical mirror images of one another or are nonsuperimposable mirror images of each other chiral - molecule that has handedness and can exist as an enantiomer achiral or nonchiral - lacking handedness, in chemistry a molecule that is identical to its mirror image, has a plane of symmetry, and can be superimposed on its mirror image. symmetry plane (plane of symmetry) - an imaginary plane that divides a molecule in half, where one half is the mirror image of the other. Objective: 1. Students will understand the three dimensional configuration of enantiomers 2. Students will be able to identify chiral and achiral molecules Introduction to Enantiomers and Handedness (High School Level) 1 Materials: -student worksheet -right hand glove -goggles Optional: -molecular stick model kits with goggles and mirror -additional examples of chiral and achiral molecules For visually impaired students use molecular models that use shapes instead spheres to represent the atoms. Procedure: 1. Begin with an introduction to what the students will be learning about in the course of this lesson. Today we will be learning about the three dimensional structure of enantiomers. Before we get started let's begin with a recap of what isomers are and their significance to chemistry (should be taught as prior lesson). Use the following leading question and followup: Who can draw an isomer of the normal butane molecule C4H10 on the board? Only one answer is possible as the example below demonstrates. 2. Follow up with an open ended question that initiates a conversation about the reactivity and properties of the isomers, for example: Are these molecules different even though they share the same formula? students may answer shape, structure, or arrangement of atoms or groups of atoms. As an open ended question ask if there is anything else that can or may be different about these isomers? Students may suggest that something other than the shape of the molecule changes as well, answers suggesting reactivity should be encouraged for elaboration by the student. Differences in chemical and physical properties of isomerism may have not been discussed in previous lessons. It is important that students understand that a seemingly minor change to the molecular shape of the molecule changes the reactivity in many cases. For these isomers here are some differences and applications to point out to the class: Introduction to Enantiomers and Handedness (High School Level) 2 n-butane - commonly used as fuel for lighters, highly flammable, boiling point of 0.5°C. Has a flat shape as compared to methylpropane. methylpropane - refrigerant and as a propellant in aerosol cans, flammable, boiling point -11.7°C. Has a spherical shape. 3. Once students agree that the shape and the configuration play a role, continue with an introduction of how isomers are classified. This lesson will focus on the branch leading to enantiomers. Use the chart below to discuss the definitions isomers, stereoisomers, and enantiomers: Explain to students that stereoisomers can be broken into two groups, enantiomers and diastereoisomers. Enantiomers are mirror image isomers and diastereoisomers are non-mirror image isomers. In this lesson we will only be examining the enantiomers. For a molecule to exist as an enantiomer is must be chiral. The human hands are chiral because it has no symmetry plane and cannot be superimposed on their mirror images. Review the following photos with the class (if possible have students replicate these photos with molecular stick models and a mirror) : Introduction to Enantiomers and Handedness (High School Level) 3 4. Review the following photo with the class. For a molecule to exist as an enantiomer is must be chiral. (if possible have students replicate these photos with molecular stick models and a mirror) : Image Source: Principles of Biochemistry 2nd ed., Cox, Lehninger, Nelson. 1993 Qualifications (tips) of Molecular Handedness: The following are some general insights that should be discussed with student to "qualify" molecules for chirality. Utilize the student worksheet, diagrams in this lesson plan or when possible molecular models and audio/video presentations. achiral molecules chiral molecules In organic compounds when carbon is In organic compounds whenever carbon bonded to two or more of the same is bonded to four different atoms or group. groups of atoms chirality is possible. Be careful, they may be identical and Has a plane of symmetry not qualify as enantiomers. Identical to its mirror image Lacks a plane of symmetry Can be superimposed on mirror image Not identical to its mirror image Cannot be superimposed on mirror image Can exist as an enantiomer Shows optical activity 5. Try this quick experiment to smell the two carvone isomers: Procedure: 1. Give each group a hand full of caraway seeds and some mint leaves 2. In one bowl or plate, crush the caraway seeds and in the other bowl crush up the mint leaves 3. Smell the crushed items from each sample and notice that the isomers have distinct smells Ask students: Describe the smell of the two items. Do you think that these molecules are the same? why or why not? As an interactive to this experiment have students see if they can figure out the similarity between the carvone molecule and fitting their right hand in a left hand glove. Introduction to Enantiomers and Handedness (High School Level) 4 Show the students the molecule of carvone. This is what they have been smelling. Have students discover how they are the same and different. Carvone has an isomer and by examining the photo they will notice that the molecules are mirror images of each other just as their hands were, so one molecule is essentially left handed (S) and the other is right handed (R). Isomers of Carvone Molecule (S) isomer "left handed" is found in caraway seed (R) isomer "right handed" is found in spearmint Both caraway and mint molecules look the same but because they are mirror images they are not exactly the same, they are isomers of carvone. The human nose has specific receptors for each kind of isomer. Imagine receptors in the nose shaped like gloves and each isomer of carvone can only fit in the corresponding glove. 6. Take some time to discuss how chirality is relevant to modern medicine and industry. The three dimensional conformation of molecules plays an important role in the world of medicine. How medicines act in the body are often specific to the shape of the molecule. In the binding of a substrate (reactant) to the catalytic (active site) of an enzyme the two molecules must fit each other closely and in a complimentary fashion. Have students try putting a left hand in a right hand glove as a demonstration of this concept. Students can also attempt to shake hands using only their right hands, even though they are both hands in their functionality and construction shaking hands with others only works one way. Additional Resources: http://www.youtube.com/watch?v=3WZZXPOsPNI http://pubs.acs.org/cen/coverstory/8023/8023chiral.html http://pubs.acs.org/cen/coverstory/7843/7843scit1.html Introduction to Enantiomers and Handedness (High School Level) 5 Student Worksheet for: Enantiomers and Handedness Name: Date: 1. Draw an isomer of the following molecule: 2. What else is likely to change among isomers other than the position of atoms or groups of atoms? _________________________________________________________________________ Isomer Classification Key: Define the following: I. Isomers: _______________________________________________________________________ II. Stereoisomers: _______________________________________________________________________ III. Enantiomers: _______________________________________________________________________ IV. Chiral: ________________________________________________________________________ V. Non-Chiral: _______________________________________________________________________ Introduction to Enantiomers and Handedness (High School Level) 6 3. Recreate the molecules in these images utilizing molecular stick models: Image Source: Principles of Biochemistry 2nd ed., Lehninger, Nelson, Cox, 1993 4. Recreate these images depicting enantiomers utilizing molecular stick models and your hands: 5. Which of the following objects are chiral? 1. a glove (yes) 2. a baseball (no) 3. a chair (no) 4. a foot (yes) Introduction to Enantiomers and Handedness (High School Level) 7 5. a pencil (no) 6. a mug (yes) 6. Using your molecular stick models recreate the following pairs of molecules. Indicate if they are identical or enantiomers. (A. identical B. enantiomer C. enantiomer) 7. Using your molecular stick model create a new enantiomer and draw your result below: 8.Research how enantiomers may play a role in a real world applications such as medicine and the drug industry? Discuss specific issues and specific molecules where possible. ______________________________________________________________________________ Introduction to Enantiomers and Handedness (High School Level) 8