What is the total number of electrons shared in
advertisement

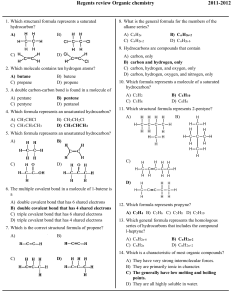
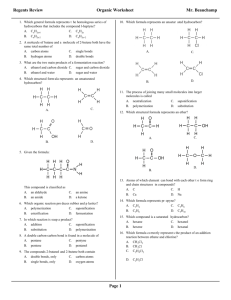
Name: _____________________________ 1) ____ Which compound is a saturated hydrocarbon? a) b) c) d) 2) ____ The isomers butane and methylpropane differ in their a) b) c) d) 3) an alpha particle a beta particle a neutron a positron ____ A beta particle may be spontaneously emitted from a) b) c) d) 5) molecular formulas structural formulas total number of atoms per molecule total number of bonds per molecule ____ Which particle has the greatest mass? a) b) c) d) 4) propanal propane propene propyne a ground-state electron a stable nucleus an excited electron an unstable nucleus ____ Which formula represents 2-butene? Base your answers to questions 6 through 8 on the information below. During a bread-making process, glucose is converted to ethanol and carbon dioxide, causing the bread dough to rise. Zymase, an enzyme produced by yeast, is a catalyst needed for this reaction. 6) Balance the equation provided for the reaction that causes bread dough to rise, using the smallest whole-number coefficients. 7) In the space provided draw a structural formula for the alcohol formed in this reaction. 7) 8) State the effect of zymase on the activation energy for this reaction. 8) 9) ____ A straight-chain hydrocarbon that has only one double bond in each molecule has the general formula a) b) c) d) CnH2n−6 CnH2n−2 CnH2n CnH2n+2 Regents review – Organic Chemistry 1-1 Created: April 2008 Name: _____________________________ 10) ____ Which reaction results in the production of soap? a) b) c) d) 11) ____ Ethanol and dimethyl ether have different chemical and physical properties because they have different a) b) c) d) 12) esterification fermentation polymerization saponification functional groups molecular masses numbers of covalent bonds percent compositions by mass ____ Which formula represents an unsaturated hydrocarbon? Base your answers to questions 13 through 17 on the information below. Biodiesel is an alternative fuel for vehicles that use petroleum diesel. Biodiesel is produced by reacting vegetable oil with CH3OH. Methyl palmitate, C15H31COOCH3, a compound found in biodiesel, is made from soybean oil. One reaction of methyl palmitate with oxygen is represented by the balanced equation below. 13) Write an IUPAC name for the compound that reacts with vegetable oil to produce biodiesel.___________________ 14) Explain, in terms of both atoms and molecular structure, why there is no isomer of CH3OH. 15) Identify the class of organic compounds to which methyl palmitate belongs. .___________________ 16) Identify the type of organic reaction represented by the balanced equation. .___________________ 17) State evidence from the balanced equation that indicates the reaction is exothermic. 18) ____ What is the empirical formula of a compound that has a carbon-to-hydrogen ratio of 2 to 6? a) b) c) d) CH3 C2H6 C3H C6H2 Given the formula for an organic compound: 19) ____ This compound is classified as an a) aldehyde b) amine c) ester d) organic acid Regents review – Organic Chemistry 2-2 Created: April 2008 Name: _____________________________ 20) ____ Butanal and butanone have different chemical and physical properties primarily because of differences in their a) b) c) d) functional groups molecular masses molecular formulas number of carbon atoms per molecule Base your answers to questions 21 through 23 on the information below. The formula below represents a hydrocarbon. 21) Identify the homologous series to which this hydrocarbon belongs. ________________ 22) Explain, in terms of carbon-carbon bonds, why this hydrocarbon is saturated. 23) In the space provided, draw a structural formula for one isomer of this hydrocarbon. Regents review – Organic Chemistry 3-3 Created: April 2008