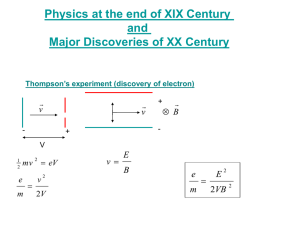
Nina A21 c100 Xie Yiling Topic electron transition and emission
advertisement

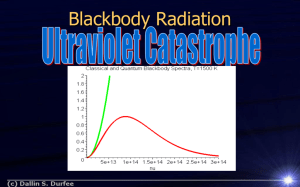
Nina c100 Xie Yiling A21 Topic electron transition and emission spectral lines/hf=E1-E2 Light quanta and the photoelectric effect The photoelectric effect occurs when a light beam is incident on a metal surface causing electrons to be ejected from the surface. The long held wave theory of light; even for light of high intensity, was inadequate to explain the phenomenon. In 1905 Albert Einstein, a little known patent office clerk, published a paper on the photoelectric effect postulating a “quantum” explanation in which light can be considered as a stream of particles called photons. This extended Planck’s quantization of energy hypothesis, which he had proposed for understanding and interpreting the experimentally obtained blackbody spectral-distribution curves of radiation power versus wavelength. These photons were to be understood as individually interacting with the electrons of the metal surface of incidence and having energy, proportional to their frequency given by Planck’s constant h and times the frequency f, (hf). With enough photon energy, that is high enough frequency to remove electrons from the metal—energy exceeding the metal’s work function; the photoelectric effect occurs with electrons being ejected from the metal. The source of photons analyzed in this experiment are emitted by light emitting diodes (LEDs), which are devices made from a class of solids called semiconductors—with electrical conductivity between that of a metal and an insulator. These light emitting diodes have p-n junctions which involve energy bands and gaps in solids. The carriers (electrons and holes) are injected across the junctions producing the light. Rather than light commonly produced from thermal excitation, the photons are emitted having rather specific energies corresponding quite closely to the energy difference, Egap, involved between the higher to lower energy bands of transition for the particular LED. Theoretically, the photoelectric effect with light incident on a metal surface and electrons ejected can be expressed by Ek=hf-W where Ek is the kinetic energy of the electrons, e is the electron unit charge, V0 is the stopping potential or voltage, h is Planck’s constant, f is the light photon frequency, and W0 is the metal work function. Energy level diagrams are a means of analyzing the energies electrons can accept and release as they transition from one accepted orbital to another. These energies differences correspond to the wavelengths of light in the discreet spectral lines emitted by an atom as it goes through de-excitation or by the wavelengths absorbed in an absorption spectrum. Notice how each energy level closer and closer to the nucleus is more and more negative. This signifies that the electron is trapped in an "energy well." To ionize a ground-state electron [to take it from -122.4 eV to 0 eV in our example], you would have to irradiate the gas with photons having energies of 122.4 eV or greater. This is the only instance where the incident energy does not have to exactly match the difference in two energy levels. Any excess energy would remain in the form of the ionized electron's kinetic energy. Max Planck had already determined that the energy levels in an oscillating system were quantized and followed the relationship E = hf where f represented the frequency of oscillation and h is Planck's constant, 6.63 x 10-34 J sec. In our case, f is the frequency of the emitted photon in accordance with c = fλ. If we replace f with c/λ, we have the formula E = h(c/λ) The value of E, or energy, represents the difference in the energies of two energy levels (ΔE) when an electron goes through de-excitation. The value of λ corresponds to the wavelength of the emitted spectral line. λ = hc/ΔE Application of electron transiton Nuclear Excitation by Electron Transition and Its Application to Uranium 235 Separation A new mechanism for nuclear excitation is studied theoretically by considering the deexcitation of electronic states of atom. When an electron of inner shells is kicked off by the bombarded electron or X ray, an electron of the adjacent shells immediately jumps into the vacancy. The energy corresponding to the difference of the binding energies for these two shells, E_1-E_2, is usually carried away by the emitted characteristic X ray or the Auger electron which is ionized from an outer shell. There is, however, another possibility of this energy release by exciting a nuclear state. That is, the nuclear ground state is excited to the higher energy level by receiving the excess energy of the electronic state by means of electromagnetic interactions between nucleus and electrons. A theory is made for this process, and it is found that the probability of this process is extremely small in most of the cases, but it will be appreciable, if the interaction energy is approximately equal to the energy mismatch E_N-(E_1-E_2), where E_N is the nuclear excitation energy. An example of 235U is discussed in connection with a possible separation of 235U from uranium isotopes. Application of photoelectric effect Applications of the photoelectric effect A photocell is a vacuum tube with a concave cathode called an emitter, made from a material that emits electrons easily. When light of a frequency greater than the threshold frequency, shines on the emitter, electrons are released. The concave emitter focuses electrons on a thin wire anode called a collector. The thin anode does not block light. When the collector is at a positve potential, electrons are attracted to it. The photocell is at the centre of the many applications of the photoelectric effect. It consists of a curved emitter and a rod as collector, so as not to inhibit light from reaching the emitter. The flash of a camera uses the photoelectric effect (for the source of this image and for more information click here) Photocells are used in garage door openers. Photocells are used in movie film. An example is shown in the diagram below: