BN-PAGE PROTOCOL
advertisement

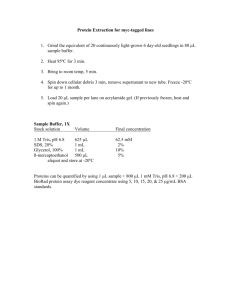
BN-PAGE PROTOCOL A) DETERMINING THE COMPLEX I STABILITY ON HEK293 CELLS B) APPARENT MOLECULAR SIZE OF DIFFERENT OXPHOS COMPLEXES IN Dictyostelium C) LOCALIZATION OF OXPHOS ASSEMBLY FACTORS FUSED TO GFP IN SUBCOMPLEXES Sergio Carilla Performing the gradient gel (A,B,C) 1. Assembly BIORAD MiniProtean3 1.5mm spacing glasses. Test there are no leaks with distilled water and remove it turning it upside down. 2. Prepare the mix in 50ml Falcon without TEMED (Sigma) nor ammonium persulfate (APS. BioRad) according the indications of table 1 (Calvaruso et al. 2008) and keep it in ice. Remember to use Acrylamide 29:1 (Biorad). 3. Use a gradient mixer (be sure there is no SDS) and keep it above the BIORAD system. Use one magnetic stirrer in the exit compartment. Insert the edge of the tube between the glass plates only a little bit (to make sure is not moving when it flows). 4. Add TEMED and ammonium persulfate to the Falcon tube according table 1 and load the two solutions (5% and 15%) into the respective compartments with 3.75 ml each one. 5. Switch on the stirrer and open the valve between compartments. Avoid bubbles. Let it to finish and add some water to let it to polymerize at RT (2 hours). 6. Invert the system to let the water go out and proceed with the stacking gel. These gels can be kept at 4ºC for weeks under a moisture atmosphere. Sample preparation and electrophoresis (A,B,C) 1. Harvest 1 x107 HEK293 or Dictyostelium cells and wash with PBS 1X in 1.5 ml protein lobind eppendorf tubes (Carilla-Latorre et al. 2010). 2. Resuspend the cells in 100 l cold PBS 1X. Add 100 l of digitonin solution (4mg/ml in PBS) 3. Incubate 10 min 4ºC to dissolve the membranes. 4. Add 1 ml of cold PBS 1X to dilute digitonin (Sigma) and centrifugate 10 min 10000g 4ºC. 5. Discard supernatant and wash the pellet with 1ml cold PBS 1X. Centrifugate 10 min 10000g 4ºC. 6. At this moment we can freeze the pellet at -80 ºC. 7. Dissolve the pellet with 100 l of gel buffer 3X. The buffer is very dense so use cut tips and try not to make bubbles. 8. Measure the protein concentration by Bradford or by BCA. 9. Add 8 l Dodecyl-maltosyde (DDM) 10% for 40 g of protein and complete to 40 l with gel buffer 3X. The quantity of protein /well can vary form 40 g (Western Blot) to 10g for the 2D BNPAGE (same final volume). 10. Vortex 15 sec every 3min during 20 min. Keep the sample on ice. 11. Centrifuge 10000g 30 min 4ºC. 12. The supernatant is transferred to another protein lobind tube and its volume measured. Add 1/10 sample buffer and load the gel. 13. Run the gel at 30 V for 30 min. Then increase the voltage to 80 V. When the front has reached at the middle of the running gel replace cathode buffer A by cathode buffer B. 14. Electrophoresis continues until front reaches the end of the gel at 80V. 15. Stain the complexes with the typical Coomassie blue protocol if you want, perform a western blot or perform the 2nd denaturing dimension. Whatever the case its interesting to use a Native Protein Marker (Invitrogen). 2nd Denaturing dimension (C) 1. Cut the 1D- lane of interest and put it perpendicular over 1mm spacing glass. Do it in extractor hood and carefully. 2. Slowly add denaturing buffer (1% SDS, 1% V/V -mercaptoethanol) over the gel lane and cover it to avoid rapid evaporation of this compound. Incubate for 30 min, change the buffer (avoid gel drying) and incubate 30 min more. 3. Eliminate mercaptoethanol remains with paper and cover it with a slim glass being carefull and mount the system. (It’s very easy to break the slim glass). 4. Be sure there are no leaks by using water. Eliminate this water turning it upside down. 5. Add the running buffer by one side of the lane (that now has been pressed between the two glasses) and let 0.5- 1 cm space with the lane. Let it to polymerize putting water above the gel. 6. Eliminate water and add the stacking solution (see Table 2). 7. Electrophoresis is started at 30V for 30 min and then is continued at 80V (2-4h). 8. Transfer it according the next section. Table 2: For 8 gels 4% stacking Acril/Bisacril (29:1)40% 1,5ml Gel buffer II 3.7ml Glicerol H2O to 15ml 10% running 1oml 13.3 ml 5,3 g to 40ml APS 10% TEMED 250l 20l 150l 15l Western blot (A,C) 1. Is carried out O/N at 30 V RT in buffer TrisGly 1X, 20 % methanol, 0.02 % SDS using a PVDF membrane. 2. After the transference introduce the membrane in stripping buffer (2%SDS, TrisHCl 62.5 mM PH 6.8) for 1.5 h to eliminate blue dye wich is an interfering agent in WB. Change the buffer 2 or 3 times until dyes fade away. 3. Block the membrane for 1.30 h with 0.5 % milk on TBST after washing 2 or 3 times with this solution. 4. Incubate with total OXPHOS Human WB Antibody Cocktail (Mitosciences) for 2h. in 0.5% TBST milk (Sigma) (A) or incubate with -GFP (Sigma) in the same solution for 1h (C). Try different concentrations for optimal results. 5. Washing steps: 2 times for 10 min with 0.5 % milk-TBST , 2 times with TBST 10 min at RT. 6. Incubate with secondary antibody goat-antimouse IgG-HRP (Santa Cruz) diluted 1/1000 in TBST or in the case of assembly factors fused to GFP with -rabbit (Santa Cruz) diluted 1/100000 in TBST 7. 3X washing steps with TBST 10 min RT. 8. Reveal with ECL (Amersham) (1 min is usually adequate). BUFFERS : Buffer 3X: 750mM aminocaproic acid, 150 mM Bis-Tris PH 7.0. Sample buffer: 750 mM aminocaproic acid (Sigma), 75 mM BisTris/HCl (Fluka)PH 7, 0.5mM EDTA, 5% Serva blue G (Serva). Anode buffer: 50mM Bis-Tris; pH 7. Cathode buffer A (1D): 50mM Tricine (Sigma), 15mM Bis-Tris, 0.02% Serva Blue G; pH 7 Cathode buffer B (1D): 50 mM Tricine, 15mM Bis-Tris; pH 7 Gel Buffer II (SDS): 3M Tris/Hcl; 0.3 % SDS; pH 8.45 Cathode buffer (2D): 0.1 M Tris; 0.1 M Tricine; 0.1 % SDS; pH 8.2 Anode buffer (2D): 0.2 M Tris/Hcl; pH 8.9 BIBLIOGRAPHY Calvaruso, M. A., Smeitink, J., and Nijtmans, L. (2008), 'Electrophoresis techniques to investigate defects in oxidative phosphorylation', Methods, 46 (4), 281-7. Carilla-Latorre, S., et al. (2010), 'MidA is a putative methyltransferase that is required for mitochondrial complex I function', J Cell Sci, 123 (Pt 10), 1674-83.