Lecture 11.
advertisement

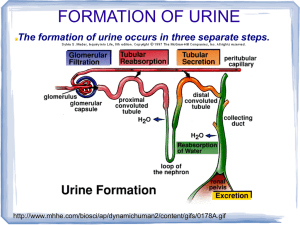
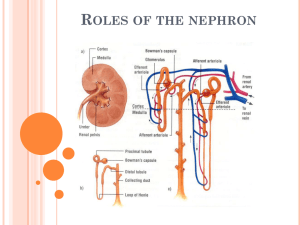
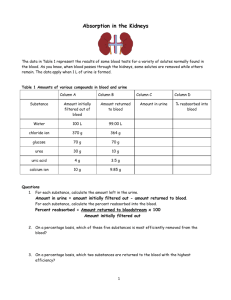
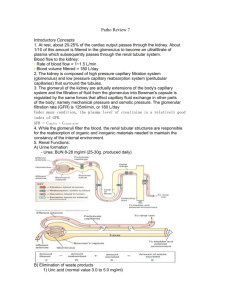
Lecture 11 PHYSIOLOGY OF THE KIDNEYS AND THE URINARY TRACT I. KIDNEY ANATOMY Pelvis Cortex Medulla: Outer medulla subdivides into an outer and inner stripe Inner medulla has papillae (tips) -> minor calyx -> major calyx Renal artery is subdivided into interlobar arteries -> arcuate artery -> cortical radial artery ->afferent arteriole ->glomerular capillary network -> efferent arteriole ->peritubular capillary network GENERAL REVIEW 1. The formation of urine involves glomerular filtration, tubular reabsorption, and tubular secretion. 2. The renal clearance of a substance is equal to its rate of excretion divided by its plasma concentration. 3. Inulin clearance provides the most accurate measure of glomerular filtration rate (GFR). 4. The clearance of p-aminohippurate (PAH) is equal to the effective renal plasma flow. 5. The rate of net tubular reabsorption of a substance is equal to its filtered load minus its excretion rate. The rate of net tubular secretion of a substance is equal to its excretion rate minus its filtered load. 6. The kidneys, especially the cortex, have a high blood flow. 7. Kidney blood flow is autoregulated; it is also profoundly influenced by nerves and hormones. 8. The glomerular filtrate is an ultrafiltrate of plasma. 9. GFR is determined by the glomerular ultrafiltration coefficient, glomerular capillary hydrostatic pressure, hydrostatic pressure in the space of Bowman's capsule, and glomerular capillary colloid osmotic pressure. 10. The proximal convoluted tubule reabsorbs about 70% of filtered Na+, K+, and water and nearly all of the filtered glucose and amino acids. It also secretes a large variety of organic anions and organic cations. 11. The transport of water and most solutes across tubular epithelia is dependent upon active reabsorption 01 Na+. 12. The thick ascending limb is a water-impermeable segment that reabsorbs Na+ 1 via a Na-K-2CI cotransporter in the apical cell membrane and a vigorous Na+/K+ATP transport in the basolateral cell membrane. 13. The distal convoluted tubule epithelium is water-imper meable and reabsorbs Na+ via a thiazide-sensitive apical membrane Na-CI cotransporter. 14. Cortical collecting duct principal cells reabsorb Na+ and secrete K+. 15. The kidneys save water for the body by producing urine with a total solute concentration (Le., osmolality) greater than plasma. 16. The loops of Henle are countercurrent multipliers set up an osmotic gradient in the kidney medulla. Vasa recta are countercurrent exchangers; they passively help maintain the medullary gradient. Collecting d are osmotic equilibrating devices; they have a low ter permeability, which is increased by arginine Vasopressin (AVP). 17. Genetic defects in kidney epithelial cells account several disorders. Nephrons are the functional units of the kidney They are superficial, juxtamedullary and midcortical. Renal corpuscle: glomerulus plus Bowman's capsule. The superficial nephron has a short loop of Henle that reaches into the outer medulla only. Its efferent arteriole branches into the peritubular capillary network that surrounds the tubular segments of the same and other nephrons. The juxtamedullary nephron arises from the deep cortical regions, next to the medulla, glomerulus is larger than that of the superficial nephron, its loop of Henle reaches into the inner medulla. Its efferent arterioles form peritubular capillary network and vasa recta. Vasa recta break into capillaries surrounding collecting ducts and the loops of Henle. Heterogeneity of nephrons Their heterogeneity is due to several various subdivisions of tubules and several types of cells in the tubules. There are different types of microvilli at the luminal surfaces, different number of mitochondria, different number of basal infoldings. Juxtaglomerular apparatus (JGA) It is formed by specialized epithelial cells in the thick ascending limb (the macula densa cells), lacis cells, and by secretory cells at the vascular pole where the afferent and efferent arterioles meet. JGA secretes renin. Glomerular capillaries - differ from systemic capillaries. Both permit free passage of small molecules, water, urea, chloride, glucose. 2 * glomerular capillaries are fenestrated with large pores which prevent the blood cells to leave the blood. Filtering pores have diameter 7.5 to 10 nm. The wall of the glomerular capillary consists of endothelium, basement membrane and epithelium of podocytes with foot processes. The basement membrane consists of three filamentous layers, lamina rara interna, lamina densa and lamina rara externa. The basement membrane is filtration barrier. Podocytes = attached to the basement membrane by pedicles, between them are filtration slits, 14 nm dia. The restrictive barrier probably does not contain pores, it may be composed of hydrated gel. Mesangial cells are smooth muscle-like contractile pericytes that surround the filtration capillaries within the glomerulus. Studies of the fine ultrastructure of the glomerulus show that the mesangial cell and the capillary basement membrane form a biomechanical unit capable of regulating filtration surface area as well as intraglomerular blood volume. Mesangial excitability enables a homeostatic intraglomerular stretch reflex that integrates an increase in filtration pressure with a reduction in capillary surface area. In addition, mesangial tone is regulated by diverse vasoactive hormones. Mesangial cells are also phagocytic. FUNCTIONS OF THE KIDNEYS The kidneys perform a variety of important functions: 1) They regulate the osmotic pressure (osmolality) of the body fluids by excreting osmotically dilute or concentrated urine. 2) They regulate the concentrations of numerous ions in blood plasma, including Na+, K+, CaH, MgH, Cl-, bicarbonate (HCO3 -), phosphate, and sulfate. 3) They play an essential role in acid-base balance by excreting H+, when there is excess acid, or HCO3 -, when there is,excess base. 4) They regulate the volume of the ECF by controlling Na + and water excretion. 5) They help regulate arterial blood pressure by adjusting Na+ excretion and producing various substances (e.g., renin) that can affect blood pressure. 6) They eliminate the waste products of metabolism including urea (the main nitrogen-containing end product of protein metabolism in humans), uric acid (an end product of purine metabolism), and creatinine (an end product of muscle metabolism). 7) They remove many drugs (e.g., penicillin) and foreign or toxic compounds. 8) They are the major production sites of certain hormones, including erythropoietin and 1,25-dihydroxy vitamin D3. 9) They degrade several polypeptide hormones, Including insulin, glucagon, and parathyroid hormone. 10) They synthesize ammonia, which plays a role in acid-base balance 3 11) They synthesize substances that affect renal blood flow and Na + excretion, including arachidonic acid derivatives (prostaglandins, thromboxane A2) and kallikrein (a proteolytic enzyme that results in the production of kinins). The four processes in the kidney are: * filtration * reabsorption * secretion * excretion RENAL CLEARANCE This is a main method to determine kidney function Clearance equation = indicates the volume of plasma cleared of a substance per unit time. UV C=------------------P C = clearance in ml/min. or ml/24 hr U = unit concentration in mg/ml V = urine volume/time in ml/min. P = plasma concentration in mg/ml Clearance of any substance may be measured, and the term is not restricted to renal function. GLOMERULAR FILTRATION * This process is rather unspecific. * The filtrate is like interstitial fluid or plasma without plasma proteins. * it is a function of renal corpuscles (glomerulus and Bowman's capsule). Composition of the filter: * The endothelium acts as a gross filter * basement membrane is a main filter * Bowman's capsule epithelium is an additional barrier, can phagocytize macromolecules. Ultrafiltration 4 = filtration of plasma water and non-protein constituents, crystalloids, from the glomerular capillaries into Bowman's space. Blood cells and colloids are left behind. Forces involved are Starling forces: * hydrostatic pressure is high and unchanged along the capillary. It averages 55 mm Hg. * Glomerular capillaries are less permeable to proteins than systemic capillaries. * Oncotic pressure increases along the capillary, but it is always lower than the filtration pressure. * Hydrostatic pressure in Bowman's capsule is higher than in tissues; This flow of fluid is higher in glomeruli than in tissues. DETERMINING GFR-STARLING FORCES The glomerular filtration rate (GFR) is the amount of fluid that filters into Bowman's capsule per unit time. GRF averages 180 L per day. - The driving force for glomerular filtration is the net ultrafiltration pressure across the glomerular capillaries. - Filtration is always favored. - GFR can be expressed by the Starling equation: GFR = Kf [ (PGC -- PBS) -( πGC - πBS)] GFR is fiItration rate across the glomerular capillaries. Kf is the filtration coefficient of the glomerular capillaries - Anionic glycoproteins line the filtration barrier and restrict the filtration of negatively charged plasma proteins. - PGC is glomerular capillary hydrostatic pressure, which is constant along the length of the capillary. It is increased by dilation of the afferent arteriole or constriction of the efferent arteriole. Increases in PGC cause increases in net ultrafiltration pressure and increases in GFR. - PBS is Bowman's space hydrostatic pressure and is analogous to Pi in systemic capillaries. It is increased by constriction of blockage of the ureters. Increase in PBS cause a decrease in net ultrafiltration pressure and a decrease in GFR. - πGC is glomerular capillary oncotic pressure. Normally, it increases along the length of the glomerular capillary because filtration of water increases the protein concentration of glomerular capillary blood. - πBS is Bowman's space oncotic pressure and is usually zero because the small amounts of filtered albumin are subsequently reabsorbed. RENAL BLOOD FLOW - it represents 25% of the cardiac output, about 100 times more than to the resting 5 muscle. - it is directly proportional to the pressure difference between the renal artery and the renal vein, - it is inversely proportional to the resistance of the renal vasculature. Measurement of renal plasma flow - renal plasma flow is measured by the clearance of para-aminohippuric acid (PAH) - PAH is both filtered by glomeruli and secreted by the renal tubules. - clearance of PAH measures effective RPF but it underestimates true RPF by 10%. - clearance does not include plasma flow to regions of the kidney that do not filter and secrete PAH. RPF CPAH [U]PAH V [P]PAH [U]PAH V RPF = CPAH = ----------------------[P]PAH renal plasma flow in ml/min or ml/24hr clearance of PAH in ml/min or ml/24hr urine concentration in mg/ml urine flow rate in ml/min or ml/24hr plasma concentration in mg/ml Measurement of RBF RPF RBF = -------------------hematocrit Regulation of GFR and RBF * GFR and RBF are regulated by paracrine, hormonal and neural mechanisms Autoregulation of GFR and RBF - is accomplished by changing renal vascular resistance as arterial pressure changes, thus maintaining a constant blood flow. In the range from 80 mm Hg to 180 Hg, change of RBF is less than 10% -> there is a change in vascular resistance. Predominant change in resistance occurs in the afferent arterioles. Hypotheses explaining autoregulation: * Myogenic hypothesis - arterial smooth muscle contracts in response to arterial blood pressure. Similar to other systemic arterioles. There are stretch - sensitive 6 ionic channels. * Tubuloglomerular feedback - the flow rate of tubular fluid is recorded by macula densa that governs the filtration rate in a single glomerulus. Control of renal circulation Autoregulation is overridden during heavy exercise, emotional stress, liver and heart failure, changes in the intake of salt, hemorrhage. Reflex control of GFR is mediated through hormones and the autonomic nervous system. * Sympathetic nerves innervate afferent and efferent arterioles, induce a constriction of both. * Renin-angiotensin-aldosterone system controls RBF through angiotensin II. * endothelium - derived relaxing factors such as nitric oxide (NO), prostaglandins. * endothelium - derived contracting factors - endothelin, thromboxane, angiotensin Measurement of GFR = clearance of inulin - inulin is filtered but neither reabsorbed nor secreted by the renal tubules. - it is a non-electrolyte, therefore not subject to a Gibbs-Donnan effect. - Its concentration in the glomerular filtrate is identical to that in plasma, but its concentration increases as water is reabsorbed in the tubules. - Its concentration is a function of reabsorption of fluid. [U]inulin x V 7 GFR = -----------------------------------[P]inulin GFR glomerular filtration rate is given in ml/min. or ml/24 hr [U]inulin urine concentration in mg/ml V urine flow rate [P]inulin plasma concentration in mg/ml - GFR may also be estimated with blood urea nitrogen (BUN) and plasma creatinine Glomerular-tubular balance = a protective mechanism through which tubular events feed back to regulate the GFR and protect the host from major NaCl losses. The macula densa senses increased NaCl delivery to the distal nephron, reduces the GFR by feeding back to the afferent arteriole via an interplay between angiotensin II and nitric oxide. Transcytosis (plasma proteins) Filtration usually excludes the plasma proteins, but some smaller proteins and hormones may be filtered. They are reabsorbed in the proximal tubule. In cells, they are digested or extruded by transcytosis. FILTRATION FRACTION Not all plasma could be filtered through the glomerulus. The filtration stops because of the sum of the hydrostatic pressure in the Bowman's capsule plus the rising oncotic pressure. - FF is the fraction of the RPF that is filtered across the glomerular capillaries - is normally about 0.20 (20% of the RPF is filtered.) - the remaining 80% leaves the glomerular capillaries by the efferent arterioles and fills the peritubular capillary circulation GFR Filtration fraction = --------------------RPF REABSORPTION AND SECRETION IN TUBULES PRINCIPLES OF EPITHELIAL TRANSPORT The cells of epithelium are polarized = they are bounded by two membranes that have different characteristics. 8 - Apical membrane faces the lumen. - Basolateral membrane lines lateral intercellular spaces and basement membrane. These two membranes are separated by the junctional complex (tight junction), a ring-like structure that surrounds each cell, forms contact between cells. Na-K-ATPase is only in the basolateral membranes, other transporters are in the apical membrane. Reabsorption may ba active or passive. Reabsorption of urea is passive. The substances may be transported by a transcellular or paracellular way, actively or passively, using also cotransport (symport) or countertransport. Reabsorbed from the glomerular filtrate are: * water * sodium * chloride * bicarbonate * glucose 99% of these substances are reabsorbed. Glucose is reabsorbed actively, by sodium co-transport. Transport maximum (Tm) curve for glucose - a reabsorbed substance *Transport proteins have been sequenced, they have 6 to 12 domains consisting of lipophilic amino acids. This allows the protein to loop back across the membrane. *There is also competitive inhibition (e.g. galactose and glucose) *Glucose is transported by a Na+- cotransporter in the proximal tubule (SGLT1) Filtered load of glucose increases in direct proportion to the plasma glucose concentration (filtered load of glucose = GFR x Pglucose). Reabsorption of glucose Na - glucose cotransport in the proximal tubule reabsorbs glucose from tubular fluid into the blood. There is a limited number of Na - glucose carriers. GLUT1 and GLUT2 are in the basolateral membrane of the proximal tubule. At plasma glucose concentrations less than 300 mg/dl (about 16.7 mmol/dl), all of the filtered glucose can be reabsorbed since there are many carriers available. At plasma glucose concentrations of 350 mg/dl (about 19.4 mmol/dl) or greater, the carriers are saturated. ->Therefore, increases in plasma concentration do not result in increased rates of 9 reabsorption. The point at which the carriers are saturated is the transport maximum (Tm). Glucose titration curve is constructed by determining the amount of glucose that is reabsorbed at increasing plasma concentrations. Glucose appears in urine when renal threshold for glucose is reached. The titration curve is characteristic of a Tm-limited transporter. Glucosuria: when plasma glucose concentration exceeds the renal threshold. Causes: * diabetes mellitus; * sometimes pregnancy - sugars are mainly lactose and galactose. * mutations in glucose transporters. The main is SGLT1, the most common Na+glucose cotransporter Excretion of glucose * At plasma concentrations below 300 mg/dl, all of the filtered glucose is reabsorbed and excretion is zero. The threshold (the plasma concentration at which glucose first appears in the urine) is approximately 300 mg/dl. * At plasma concentrations above 350 mg/dl (Tm), reabsorption is saturated. Therefore, as plasma concentration increases, the additional filtered glucose cannot be reabsorbed and is excreted in the urine. Splay - is the region of the glucose curves between threshold and Tm . - occurs approximately between plasma glucose concentrations of 300 mg/dl and 350 mg/dl. - represents spilling of glucose in urine before saturation (Tm) is fully achieved. - is explained by heterogeneity of nephrons and the relatively low affinity of the glucose carriers. * Splay is an area of the curve where glucose is spilled into urine before Tm is reached. It is explained by the kinetics of the chemical reaction, a saturating concentration of glucose is needed to occupy all sites for glucose on the carrier. Penicillin secretion The kidneys secrete penicillin, and within 3 - 4 hours, about 80% of the dose is excreted by urine. Probenecid slows down the secretion, it is a competitor. 10 PASSIVE REABSORPTION - UREA The rate of reabsorption of urea and similar substances is proportional to the concentration difference of a solute between the tubular fluid and the plasma, and the permeability of the tubular epithelium to the substance. It depends on * the rate of excretion by the glomerulus; * flow of the tubular fluid; It is reabsorbed by facilitated diffusion. There is a saturable carrier that is enhanced by the ADH. It is in the inner medullary collecting duct. - Fifty percent of the filtered urea is transported passively in the proximal tubule - The distal tubule and cortical and outer medullary collecting ducts are impermeable to urea, so no urea is reabsorbed by these segments. - The antidiuretic hormone increases the urea permeability of the inner medullary collecting ducts. Urea reabsorption from inner medullary collecting ducts contributes to urea recycling in the medulla. BIDIRECTIONAL TRANSPORT Some substances may be filtered, reabsorbed, but also excreted by the tubule. Tubular reabsorption is then a sum of fluxes in both directions. *Some substances undergo a net excretion in one part of the nephron, and net movement in opposite direction in another part. K+ is reabsorbed in proximal tubule and loops of Henle, secreted in distal tubules and cortical collecting ducts and reabsorbed in medullary collecting ducts. RELATIVE CLEARANCES OF SUBSTANCES 1. Substances with the highest clearances - are those that are both filtered across the glomerular capillaries and secreted from the peritubular capillaries into urine (e.g., PAH). 2. Substances with the lowest clearances - are those that are either not filtered (e.g., protein) or are filtered and subsequently reabsorbed from urine into peritubular capillary blood (e.g., Na, glucose, amino acids, HCO3-, Cl- ). ' 3. Substances whose clearance equals GFR - are those that are freely filtered but not reabsorbed or secreted (e.g., inulin ). 4. Relative clearances - PAH > K+ ( high K+ diet) > inulin :< urea > Na > glucose, amino l acids, HCO311 NA+ REGULATION Basic Rules: * water moves toward osmotic equilibrium across cell membranes. Because Na+ ions are restricted primarily to ECF, the content of Na+ in th body determines the ECF volume. * Sodium and the accompanying anions account for more than 90% of the ECF osmoles. * an ultrafiltrate moves between the vascular and interstitial spaces. This movement is controlled by the hydrostatic pressure and colloid osmotic pressure. * the body regulates the content of Na+ and water in an independent fashion. * water balance is the result of an interplay of thirst and ADH. * the kidney controls the content of Na+ in the body. * control of renal excretion of Na+ is the major way to regulate the content of Na+ in the body. * Changes in circulating volume signal the retention or excretion of Na+. * filtration and reabsorption are linked, the right amount of sodium is excreted because of glomerular - tubular balance. * ARP 2000: Dopamine is a key signal in the regulation of sodium metabolism. Natriuretic and anti-natriuretic factors released from the renal and extrarenal sources may regulate sodium exchange through dopamine receptors. Dopamine acts as a natriuretic factor. Single nephron terminology - Tubular fluid (TF) is urine at any point along the nephron. - plasma (P) refers to systemic plasma. 1. TF/Px ratio - compares the concentration of a substance in tubular fluid at a point along the nephron to its concentration in systemic plasma. For example, if TF/PNa = 1.0, then the Na+ concentration in tubular fluid is identical to the [Na+] in plasma. a. If TF/P = 1.0, then reabsorption has been exactly proportional to the reabsorption of water. b. If TF/P < 1.0, then reabsorption of the substance has occurred to a greater extent than the reabsorption of water thus making the concentration in tubular fluid fall below that in plasma. c. If TF/P> 1.0, either reabsorption of the substance has been less than reabsorption of water or there has been secretion of the substance. d. TF/Pinulin is used as a marker for water reabsorption along the nephron. Since inulin is freely filtered, but not reabsorbed or secreted, its concentration in tubular 12 fluid is determined solely by how much water remains in the tubular fluid. - TF/Pinulin increases as water is reabsorbed. For example, if 50% of the filtered water is reabsorbed, the TF/Pinulin = 2.0. B. Na+ reabsorption - review - Na+ is freely filtered across the glomerular capillaries; the [Na+] in the tubular fluid in Bowman's space equals that in plasma (TF/PNa+ = 1.0) - Na+ is reabsorbed all along the nephron and very little is excreted in urine. 1. Proximal tubule - reabsorbs 2/3 or of the filtered Na+ and H2O, more than any other part of the nephron. - is the site of glomerulotubular balance. - Reabsorption of Na+ and H2O in the proximal tubule are exactly proportional - the process is isosmotic. Therefore, both TF/PNa+ and TF/Posm = 1.0 - Reabsorption is divided into early and late proximal tubule. a. Early proximal tubule - special features - reabsorbs Na+ and H20 with HCO3-, glucose, amino acids, phosphate and lactate - part of transport is passive, depends on the electrochemical gradient. - Na+ is reabsorbed by cotransport with glucose, amino acids, phosphate, and lactate. These cotransport processes account for the reabsorption of all the filtered glucose and amino acids. - Na+ is also reabsorbed by countertransport as Na+ - H+ exchange. Na+- H+ exchange is linked directly to the reabsorption of filtered HCO3- . - Carbonic anhydrase inhibitors (e.g., acetazolamide) are diuretics that act in the early proximal tubule by inhibiting HCO3- reabsorption. Once inside the cells, sodium is transported actively on the basolateral membrane by a sodium pump into the peritubular fluid. Glucose and other substances diffuse from the tubular cells passively, by facilitated diffusion. b. Middle and late proximal tubules - special features - The reabsorption of glucose and other substances is completed at the end of early tubule, cotransport is no longer possible. - passive movement of sodium depends mainly on Na+/H+ exchanger. - In these segments, Na+ is reabsorbed with CI-. - transport from the cells depends on sodium pump. Water transport is mainly osmotic. c. Glomerulotubular balance in the proximal tubule 13 - maintains reabsorption of a constant fraction ( 2/3) of the filtered Na+ and H2O. (I) For example, if GFR increases, the filtered load of Na+ increases. Glomerulotubular balance functions such that Na+ reabsorption will also increase, ensuring that a constant fraction is reabsorbed. (2) The mechanism of glomerulotubular balance resides in Starling forces, which alter Na+ and H2O reabsorption in the proximal tubule. - The route of isosmotic fluid reabsorption is from the lumen, to the proximal tubule cell, to the lateral intercellular space, and the to the peritubular capillary blood. - Starling forces in the peritubular capillary blood govern how much of this isosmotic fluid will be reabsorbed. Fluid reabsorption is favored by increases in πc of the peritubular capillary blood.Thus, increases in GFR cause the protein concentration and πc of peritubular capillary blood to increase, which, in turn, produces an increase in fluid reabsorption - matching of filtration and reabsorption ( glomerulotubular balance). d. Effects of ECF volume on proximal tubular reabsorption (1) ECF volume contraction increases reabsorption. Volume contraction increases peritubular capillary protein concentration and πc and decreases peritubular capillary Pc. Together, these changes in Starling forces in peritubular capillary blood cause an increase in proximal tubular reabsorption. (2) ECF volume expansion decreases reabsorption. Volume expansion decreases peritubular capillary protein concentration and πc, and increases Pc. Together, these changes in Starling forces in peritubular capillary blood cause a decrease in proximal tubular reabsorption. Thin descending limb of the loop of Henle - enables the excretion of a concentrated or diluted urine. - is highly permeable to water, about 20% of water is reabsorbed. This water moves in response to hyperosmolality of interstitium arising from the countercurrent system. - is moderately permeable to Na+ and CI- and to urea, but these solutes are secreted into this segment. Thick ascending loop - is permeable to Cl- , but also to Na+, their reabsorption is passive. Water cannot follow because this segment is impermeable to water. Thick ascending limb - The Ioop of Henle reabsorbs 20 - 25% of the filtered Na+. - The transport mechanism is the Na+-K+-2CI- cotransporter in the luminal 14 membrane of cells in the thick ascending limb. - is the site of action of the loop diuretics (furosemide, ethacrynic acid, bumetanide), which inhibit the Na + - K + -2CI- cotransporter. - is impermeable to water, so NaCl is reabsorbed without water. As a result, tubular fluid [Na+ ] and tubular fluid osmolarity decrease below that of plasma (TF/PNa+ and TF/Posm < 1.0). This segment, therefore, is called the diluting segment. 3. Distal tubule and collecting duct - together reabsorb 12% of the filtered Na+. a. Early distal tubule - special features - is called the cortical diluting segment. 15 - reabsorbs NaCl by a Na +- CI- cotransporter, passive entry into the cell, sodium pump transports sodium out of the cell, chloride goes out passively via a channel. - is the site of action of thiazide diuretics (hydrochlorothiazide). - is impermeable to water, as is the thick ascending limb. Thus, the reabsorption of NaCl without water further dilutes the tubular fluid. b. Late distal tubule and collecting duct - special features - have two cell types. (1) Principal cells reabsorb Na+ and H20 and secrete K+. - Principal cells are the site of action of the following hormones or drugs: (a) Aldosterone increases Na+ reabsorption and increases K+ secretion. Like other steroid hormones, the action of aldosterone takes several hours to develop because new protein synthesis is required. About 2% of overall Na+ reabsorption is affected by aldosterone. (b) ADH increases water permeability by directing the insertion of H20 channels in the luminal membrane. In the absence of ADH, the principal cells are virtually impermeable to water. (c) K+ -sparing diuretics ( spironolactone, triamterene, amiloride decrease K+ secretion. Sodium moves into principal cells passively, through a channel, down both a chemical and electrical gradient. Sodium pump pumps sodium out of the cell . (2) Intercalated cells secrete H+ and reabsorb K+. - Aldosterone increases H+ secretion (in addition to its action on the principal cells). Sodium handling in the collecting ducts. The major function of the cortical collecting ducts is sodium reabsorption and potassium and H+ secretion. There is a sodium channel controlled by aldosterone. * in the medulla, there is concentration of urine, NH4- secretion, and sodium balance. Here, final decision about urine concentration is made. Control of sodium excretion * ECF volume = when contracted, urine is free of sodium. * anions in the urine * need to excrete NH4* excretion is controlled by hormones, angiotensin II - aldosterone - atrial natriuretic hormone K+ REGULATION * Potassium plays a key role in the generation of the resting membrane potential. 16 * The surpluses and deficits of potassium may predispose the patient to cardiac arrhythmias. A. Shifts of K+ between the ICF and ECF - most of the body's K+ (98%) is in the ICF. - a shift of K+ out of cells causes hyperkalemia. - a shift of K+ into cells causes hypokalemia. - when faced with a load of K+, entry of K+ into cells is rapid. This shift is the body's initial defense mechanism. - these shifts are influenced by insulin, β-adrenergics, aldosterone B. Renal regulation of K+ balance - K+ is filtered, reabsorbed, and secreted. - K+ balance is achieved when urinary excretion of K+ exactly equals intake of K+ in the diet. - K+ excretion can vary widely from 1% to 110 % of the filtered load, depending on dietary K+ intake, aldosterone levels, and acid-base status. 1. Filtration - occurs freely across the glomerular capillaries - TF/PK - in Bowman's space is 1.0. 2. Proximal tubule - reabsorbs 67% of the filtered K+ along with Na+ and H2O. 3. Thick ascending limb of the loop of Henle - reabsorbs an additional 20% of the filtered K+. - reabsorption involves the Na + -K + -2CI- cotransporter in the luminal membrane cells in the thick ascending limb. 4, Distal tubule and collecting duct * either reabsorb or secrete K+, depending on dietary K+ intake. * virtually all regulation of urinary K+ occurs in the cortical collecting duct (CCD). a. Reabsorption of K+ - occurs only on a low K+ diet (K+ depletion). Under these conditions, K+ excretion can be as low as I% of the filtered load as the kidney tries to conserve as much K+ as possible. - The mechanism involves a H+-K+ ATPase in the intercalate cells - aldosterone participates 17 b. Excretion of K+ - the kidneys adjust overall K+ homeostasis by increasing or decreasing the rate of excretion of K+. - is variable and accounts for the wide range for urinary K+ excretion - depends on factors such as dietary K+, aldosterone levels, acid-base status, and urine flow rate. - occurs in the principal cells. (1) Mechanism of distal K+ secretion * Active transport of K+ into the cell by the Na+-K+ pump on the basolateral membrane. This mechanism keeps the intracellular K+ concentration high. * Passive secretion of K+ into the lumen. The magnitude of this passive secretion is determined by the chemical and electrical driving force on K+ across the luminal membrane. Maneuvers that increase the intracellular K+ concentration or decrease the luminal K+ concentration will increase K+ secretion. (2) Factors that change distal K+ secretion - Distal K+ secretion is increased when the electrochemical driving force for K+ across the luminal membrane is increased. Secretion is decreased when the driving force is decreased. (a) Dietary K+ - A diet high in K+ increases K+ secretion, and a diet low in K+ decreases K+ secretion. - On a high K+ diet, intracellular K+ increases so that the driving force for K+ secretion also increases. - On a low K+ diet, K+ secretion is (by the same reasoning) decreased. In fact, on a low K+ diet, the intercalated cells are actually stimulated to reabsorb K+. (b) Aldosterone - hyperkalemia and ECF volume contraction (via angiotensin II) are the two major stimuli for aldosterone secretion. - increases K+ secretion by increasing Na+ entry into cells across the luminal membrane and increasing pumping of Na+ out of the cells by the Na+ - K+ pump. - Stimulation of the pump simultaneously increases K+ uptake into distal cells, increasing the intracellular K+ concentration and the driving force for K+ secretion. Thus, hyperaldosteronism increases K+ secre tion and causes hypokaemia - Hypoaldosteronism decreases K+ secretion and causes hyperkalemia. (c) Acid-base 18 - Acidosis decreases K+ secretion, and alkalosis increases K+ secretion. Effectively, H+ and K + exchange for each other across the cell membrane. - In acidosis, because there is excess H+ in blood, H+ leaves the distal cell in exchange for K+ leaving the cell. As a result, intracellular K + concentration decreases and the driving force for K + secretion decreases. --In alkalosis, because there is a deficit of H + in blood, H+ leaves the cell in exchange for K+ entering the cell. As a result, intracellular K+ concentration increases and the driving force for K+ secretion increases. RENAL REGULATION OF PHOSPHATE - Eighty-five percent of the filtered phosphate is reabsorbed in the proximal tubule by Na+ -phosphate cotransport. Because the distal segments of the nephron do not reabsorb phosphate, 15% of the filtered load is excreted. - parathyroid hormone (PTH) inhibits phosphate reabsorption in the proximal tubule. Therefore, PTH causes phosphaturia and increased urinary cAMP. - Phosphate is a urinary buffer for H+; excretion of H2P04 - is called titratable acid. RENAL REGULATION OF CALCIUM - Sixty percent of the plasma Ca2 + is filtered across the glomerular capillaries. - Together, the proximal tubule and thick ascending limb of the loop of Henle reabsorb 90% of the filtered Ca2+ by passive processes that are coupled Na+ reabsorption. - the distal tubule and collecting duct, together, reabsorb 9% of the filtered Ca2+ by an active process. 1. PTH increases renal Ca2+ reabsorption by activating adenylate cyclase in the distal tubule. 19