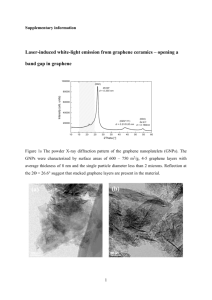
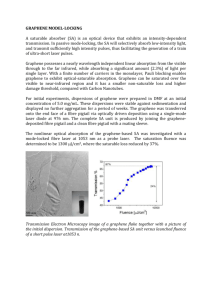
srep02144-s1
advertisement

Supplementary Information Large-scale and Rapid Synthesis of Disk-Shaped and Nano-Sized Graphene Chunyong He†, San Ping Jiang*,‡, Pei Kang Shen*,† † State Key Laboratory of Optoelectronic Materials and Technologies, and Guangdong Province ey Laboratory of Low-carbon Chemistry & Energy Conservation, School of Physics and Engineering, Sun Yat-sen University, Guangzhou, 510275, PR China ‡ Fuels and Energy Technology Institute & Department of Chemical Engineering, Curtin University, Perth, WA6102, Australia * e-mail: stsspk@mail.sysu.edu.cn (P.K. Shen); s.jiang@curtin.edu.au (S.P. Jiang) Synthesis of disk-shaped and nano-sized graphene (DSNG) The ion-exchange resins are insoluble matrixes (or support structure) normally in the form of small (1–2 mm diameter) beads, fabricated from an organic polymer substrate. The highly developed pore structure of the ion-exchange resins on the surface provides sites which can easily trap and release ions, simultaneously, the process is called ion exchange. Based on the functional groups, resins are classified into four basic categories: strong acid cation (SAC), weak acid cation (WAC), strong base anion (SBA) and weak base anion (WBA). D113 resin belongs to weak acid cation exchange resins, which derives its exchange activity from a carboxylic group (-COOH). Scheme S1 is the typical profile of the ion exchange process. As illustrated by the exchange reactions, D113 resin readily accepts cobalt ions by exchange hydrogen ions during the ion exchange process. The exchanged ion then diffuses out of the resin and back into the solution at opposite direction.S1 Scheme S1 The typical profile of the ion exchange process of D113 between Co2+ and H+. The D113 resin (Shanghai Hualing Co. Ltd., China) was pre-treated before use by a procedure as follows. The D113 resin was firstly washed with deionized water and then soaked in a solution of mixed 1.0 mol L-1 NaClO and 1.0 mol L-1 HCl for 24 h in order to remove impurities and to activate. The pre-treated resin was washed with deionized water and dried at 80 oC. In a typical preparation process, 2.0 g CoCl2.6H2O were dissolved in 200 ml deionized water and mixed with 20 g D113 resin (the mass ratio of CoCl2 .6H2O to D113 resin: RCo/R = 0.1). After magnetic stirring for 2 h, the metal ions exchanged D113 resin was rinsed with deionized water for several times and dried in oven at 80 oC. After carbonization in tubular furnace at 400 oC under Ar atmosphere condition for 2 h, the samples were heat treated at 1300 oC for 1 h. The product was post-treated in 3.0 mol L−1 HCl solution for more than 12 h with magnetic stirring to remove cobalt. Effect of synthesis conditions on the morphology of DSNG The effect of synthesis conditions such as the mass ratio of CoCl2.6H2O to D113 resin on the morphology of graphene was investigated. In this experiment, 4.0 g, 8.0 g, 20 g CoCl2.6H2O were dissolved in 200 ml deionized water and added 20 g D113 resin (RCo/R = 0.2, 0.5 and 1), respectively. After treated by the same steps as mentioned above, graphene samples with different morphologies were obtained. Figure S1 shows the graphene sample synthesized by the same steps as mentioned above but without carbonizing treatment in tubular furnace at 400 oC under Ar atmosphere for 2 h. The morphology and structure of the graphene samples are highly irregular with no formation of disk-shape graphene. Figure S1. Tapping mode AFM image of the graphene-like sheets deposited on new-delaminated mica at ambient conditions without carbonizing treatment in tubular furnace at 400 oC under Ar atmosphere for 2 h. Additional characterization and analysis results Figure S2 shows the XRD pattern of the amorphous structure of the compound of amorphous carbon(a-C) and cobalt after theD113resins located metal ions were carbonized (pattern a). The pattern b shows clearly that graphitized carbon and polycrystalline cobalt particles have been formed under the process of heating condition. The diffraction peak at 26.2°, 42.2°, 44.4°, 54.0°, and 77.2° is the characteristic of the graphite (002), (100), (101), (004) and (110) facets. The peaks at the 2θ of 45.9°, 51.5°, 75.8°, 92.2° and 97.6°. Those peaks are corresponding to the (111), (200), (220), (311), (222) facets of Co, showing the formation of polycrystalline cobalt particles.S2 As shown in Figure S2 (pattern c), the diffraction peaks of cobalt disappeared after post-treated by 3.0 M HCl for 12 h. This means that the cobalt has been removed after the hydrochloric acid treatment. Intensity / a. u. c Co Graphite b a 10 20 30 40 50 60 70 80 90 100 110 2 theta Figure S2. (a) The XRD pattern of the amorphous structure of the compound of amorphous carbon(a-C) and cobalt after the D113 located metal ions were carbonized. (b) The XRD pattern of Co/DSNG composite. (c) The XRD pattern of DSNG. Figure S3 shows the Raman spectra of the graphene samples with different value of RCo/R. All the Raman spectra of the graphene samples show the characteristics of graphene. G band Negative shift 20cm 2D band -1 D band Intensity / a. u. RCo/D113=0.2 RCo/D113=0.5 RCo/D113=1 HOPG 1000 1500 2000 2500 Raman shift / cm-1 3000 Figure S3. Raman spectra at 514 nm for the graphene samples with different RCo/R and HOPG. The 2D band in the Raman spectrum obtained from graphene is formed by one Lorentzian peak. Figure S4 shows the high-resolution C1s XPS spectra of the graphene samples with different value of RCo/R and HOPG. The C1s peak can be fitted by one Lorentzian peaks at 284.6, corresponds to the graphite-like sp2 bonded carbon. The C1s spectrum of graphene samples coincides with HOPG, indicating all the carbon atoms of graphene samples are arranged in a conjugated honeycomb lattice. Intensity / a.u. C1s 294 RCo/R = 0.2 RCo/R = 0.5 RCo/R = 1 HOPG 292 290 288 286 284 282 280 Binding energy / eV Figure S4. High-resolution C1s XPS spectra of the graphene samples with different value of RCo/R and HOPG. To further confirm the DSNG is formed in the process of heat treatment, instead of in the process of acid treatment, we take AFM test for the Co/graphene composite sample without acid treatment. In the case of the RCo/R equals 0.1, as seen from the inhomogeneous height color of the AFM image in Figure S5a, there are obviously two types of morphologies: the light yellow of the DSNG and the thick yellow in the inner circle, which is certainly the cobalt particle on the top surface of the DSNG. When the RCo/R increases to 0.2, we obtained well-shaped cobalt disks (Figure S5b). Increasing the RCo/R to 0.5, we obtained DSNGs with different sizes of the cobalt particles (Figure S5c), consequently, different sizes of the DSNGs. When the RCo/R reaches 1 (Figure S5d), the cobalt particles still located on the surface of the DSNGs, however, the cobalt particles become irregular in shape and size and finally the DSNGs as well. Figure S5. Tapping mode AFM images of Co/graphene (without acid treated) deposited on on new-delaminated mica at ambient condition. (a) RCo/R = 0.1, (b) RCo/R = 0.2, (c) RCo/R = 0.5, (d) RCo/R = 1. Figure S6 shows the tapping mode AFM images of the Co/graphene (without acid treatment) samples deposited on new-delaminated mica at ambient condition as a function of mass ratio of CoCl2 .6H2O to D113 resin. The height profiles indicated that the relative heights are 0.78 nm, 0.71 nm, 0.44 nm and 0.72 nm corresponding to the different RCo/R. Figure S6. Tapping mode AFM images of the Co/graphene (without acid treatment) samples deposited on new-delaminated mica at ambient condition as a function of mass ratio of CoCl2 .6H2O to D113 resin: (a) RCo/R = 0.1, (b) RCo/R = 0.2, (c) RCo/R = 0.5 and (d) RCo/R = 1. (a1-d1) are the height profiles along the blue lines in (a-c), respectively. The folded edges of a-c exhibit relative heights of 0.78 nm, 0.71 nm, 0.44 nm and 0.72 nm, respectively. Figure S7 Photograph of suspension of DSNG (1 mg ml− N-methyl-2-pyrrolidone 1,2-Dichloroethane (EDC). (NMP), N,N-Dimethylformamide 1 ) in H2O, (DMF) and Normalized PL intensity 1.0 0.8 0.6 0.4 large flake graphene 0.2 0.0 100 1000 10000 100000 Graphene size / nm Figure S8 The relationship of the normalized PL intensity with the graphene size. The origin of photoluminescence (PL) in these carbon-based nanomaterials is tentatively proposed to be from isolated polyaromatic structures or passivated surface defects.S15 For the PL mechanism of functionalized graphene, it is speculated that the size effect, shape, fraction of the sp2 domains, and surface defects contribute to PL.S12-14,S16 So the PL performance of functionalized graphene is decide by the size, shape, defects, and preparation method. Table S1 displays different examples of the photoluminescence from functionalized graphene. Details on fabrication method, lateral size of the flakes, and PL performanc are included. Table S1. The photoluminescent of functionalized graphene. Size Samples nm / PL Preparation method Excited emission wavelength References peak / / nm nm GO 752 500 S3 570 400 S4 321 280 S5 440 325 S6 Modified Hummers 520 400 S4 321-421 280 S5 Reduce GO 390 325 S6 Micro-wave-assiste 303 197 S7 427 320 S8 430 320 S9 430 325 S10 Hummers method 20 Nano-GO method Reduced GO 3.4 d hydrothermal 5.3 Solvothermal method Graphene quantum (GQDs) 9.6 dots Hydrothermal approach 5-15 Hydrothermal approach 15 Bottom–up method 460 365 S11 5-35 Ultrasound-assisted 300-470 325 S12 solvent method 60 Chemical process 430-560 320-480 S13 plasma 700 nm 473 S14 270 Our work from HBC Oxygen plasma Oxygen treated treatment graphene Disk-shaped 200 nanographene Pyrolysis of D113 311 resin References: S1. Barbaro, P.; Liguori, F. Chem. Rev. 2009, 109, 515. S2. Wang, S. M.; Pei, Y. H.; Wang, X.; Wang, H.; Meng, Q. N.; Tian, H. W.; Zheng, X. L.; Zheng, W. T.; Liu, Y. C. J. Phys. D: Appl. Phys. 2010, 43, 455402. S3 Luo, Z.; Vora, P. M.; Mele, E. J.; Johnson, A. T. C.; Kikkawa, J. M. Appl. Phys. Lett. 2009, 94, 111909. S4 Sun, X.; Liu, Z.; Welsher, K.; Robinson, J. T.; Goodwin, A.; Zaric, S.; Dai, H. Nano Res. 2008, 1, 203. S5 Xin, G.; Meng, Y.; Ma, Y.; Ho, D.; Kim, N.; Cho, S. M.; Chae, H. Mate. Lett. 2012, 74, 71. S6 Eda, G.; Lin, Y.-Y.; Mattevi, C.; Yamaguchi, H.; Chen, H.-A.; Chen, I. S.; Chen, C.-W.; Chhowalla, M. Adv. Mater. 2010, 22, 505. S7 Zhu, S.; Zhang, J.; Qiao, C.; Tang, S.; Li, Y.; Yuan, W.; Li, B.; Tian, L.; Liu, F.; Hu, R.; Gao, H.; Wei, H.; Zhang, H.; Sun, H.; Yang, B. Chem. Commun. 2011, 47, 6858. S8 Gupta, V.; Chaudhary, N.; Srivastava, R.; Sharma, G. D.; Bhardwaj, R.; Chand, S. J. Am. Chem. Soc. 2011, 133, 9960. S9 Pan, D.; Zhang, J.; Li, Z.; Wu, M. Adv. Mater. 2010, 22, 734. S10 Tang, L. B.; Ji, R. B.; Cao, X. K.; Lin, J.Y.; Jiang, H. X.; Li, X. M.; Teng, K. S.; Luk, C. M.; Zeng, S. J.; Hao, J. H.; Lau, S. P. ACS Nano 2012, 6, 5102. S11 Liu, R.; Wu, D.; Feng, X.; Müllen, K. J. Am. Chem. Soc.2011, 133, 15221. S12 Kim, S.; Hwang, S. W.; Kim, M. K.; Shin, D. Y.; Shin, D. H.; Kim, C. O.; Yang, S. B.; Park, J. H.; Hwang, E.; Choi, S-H.; Ko, G.; Sim, S.; Sone, C.; Choi, H. J.; Bae, S.; Hong, B. H. ACS Nano, DOI: 10.1021/nn302878r. S13 Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Carbon 2012, 50, 4738. S14 Gokus,T.; Nair, R. R.; Bonetti, A.; Böhmler, M.; Lombardo, A.; Novoselov, K. S.; Geim, A. K.; Ferrari, A. C.; Hartschuh, A. ACS Nano 2009 3, 3963. S15 Mei, Q.; Zhang, K.; Guan, G.; Liu, B.; Wang, S.; Zhang, Z. Chem. Commun. 2010, 46, 7319. S16 Robertson, J. Phys. Rev. B 1996, 53, 16302.