Microsoft Word for Windows Template
advertisement

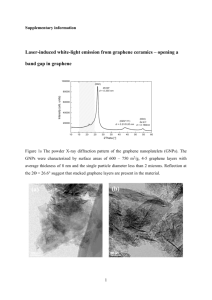
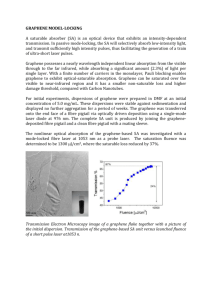
On the Intrinsic Wettability of Graphene Haitao Liu1, Zhiting Li1, Yongjin Wang2, Andrew Kozbial2, Lei Li2 1 Department of Chemistry, University of Pittsburgh, 2 Departmetn of Chemical and Petroleum Engineering, University of Pittsburgh, Pennsylvania, USA Graphene and graphite are long believed to be hydrophobic. Here we show that a clean graphitic surface is in fact mildly hydrophilic [1]. We find that an as-prepared graphene sample is hydrophilic with a water contact angle of ca. 40º. Upon exposure to ambient air, the water contact angle gradually increased to ca. 60º within 20 min and plateaued at ca. 80º after 1 day (Fig 1a). Infrared (IR) spectroscopy and X-ray photoelectron spectroscopy (XPS) showed that airborne hydrocarbon adsorbed onto the graphene surface during this process (Fig 1b). Both thermal annealing and controlled UV/O3 treatment removed the hydrocarbon contaminants, which was accompanied by a concurrent decrease in the water contact angle. Our findings show that graphene is more hydrophilic than previously believed and suggest that the reported hydrophobic nature of graphene is due to unintentional hydrocarbon contamination from ambient air. Fig. 1. (a) Temporal evolution of the WCA measured on a graphene/copper sample. The sample was taken out of the CVD chamber at time 0. The three photographs show the water drops captured at 1 min, 60 min, and 1200 min. (b) ATR-FTIR spectrum of a graphene/copper sample. The sample was taken out of the CVD chamber at time 0. The inset shows the integrated peak area vs time for the peaks at 2930 cm-1 (asymmetric CH2 stretching, blue) and 2850 cm-1 (symmetric CH2 stretching, red). References [1] Zhiting Li, Yongjin Wang, Andrew Kozbial, Ganesh Shenoy, Feng Zhou, Rebecca McGinley, Patrick Ireland, Brittni Morganstein, Alyssa Kunkel, Sumedh P. Surwade, Lei Li & Haitao Liu, Nature Materials, 12, 925-931, (2013).