Determining Molar Mass by Freezing Point Depression
advertisement

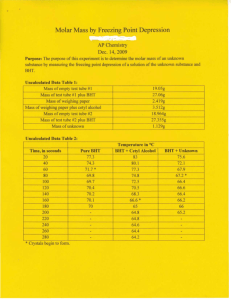
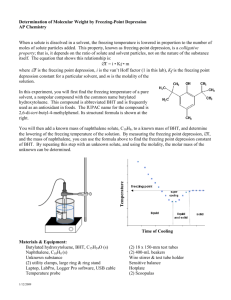
Determining Molar Mass by Freezing Point Depression Experiment Overview: The purpose of this experiment is to determine the molar mass of an unknown substance by measuring the freezing point depression of a solution of the unknown substance and 2,6-Di-tert-butyl-4-methylphenol (BHT). The freezing point of BHT is first determined. Then, a known amount of cetyl alcohol is then added to a measured quantity of BHT. The freezing point depression of this solution is found and the freezing point depression constant (kfp) is calculated. The unknown is added to the BHT, the freezing point depression of this solution is measured, and the molar mass of the unknown is then determined. Materials: BHT hot plate Cetyl Alcohol rubber stopper Unknown substance thermometer Ring stand stirrer Universal clamp Capillary tube with rubber band Safety: BHT, cetyl alcohol and the unknown are moderately toxic by ingestion and inhalation and are body tissue irritants. Wear chemical splash goggles. Pre-Lab: 1. Draw the structural formula of BHT in the space below. What is the formula for Cetyl Alcohol? The following data were obtained in an experiment designed to find the molar mass of a solute by freezing point depression. Solvent: para-dichlorobenzene Freezing point depression constant: 7.1 °C/m Freezing point of pure solvent: 53.02 °C Mass of para-dichlorobenzene: 24.80 g Mass of unknown substance: 2.04 g Freezing point of solution: 50.78 °C 1. Determine the freezing point depression, ∆Tfp. 2. Using the equation below, calculate the molar mass of the unknown substance: Molar Mass (solute) = kfp x g(solute) Kg(solvent) x ∆Tfp Procedure: 1. Pack BHT in a capillary tube to a depth of about 1 cm. 2. Obtain a small rubber band, and use it to fasten the capillary tube to a thermometer. The BHT should be level with the bulb of the thermometer. 3. Use a universal clamp and split rubber stopper to fasten the thermometer to a ring stand. 4. Immerse the bottom of the capillary and thermometer in a beaker of water and heat. 5. Stir the water in the beaker to obtain an even distribution of temperature. Heating can be very rapid in the beginning so heat slowly in order to get an accurate value. 6. Record the temperature at which the BHT melts (the white powder will become clear) in Data Table #1. 7. Repeat steps 1-6 with a sample of the BHT + Cetyl Alcohol (Solution #1). 8. Repeat steps 1-6 with a sample of the BHT + Unknown (Solution #2). 9. Dispose of the samples in the appropriate waste container Data and Analysis: Melting Points - Data Table #1 Melting Point Pure BHT BHT + Cetyl Alcohol BHT + Unknown Masses - Data Table #2 BHT Cetyl Alcohol Unknown Solution #1- BHT + Cetyl Alcohol,g Solution #2- BHT + unknown, g Conclusion: 1. Determine the ∆Tfp for the solution of cetyl alcohol and of the unknown substance in BHT. 2. Determine the freezing point constant, Kfp, for BHT. 3. Use the calculated value of Kfp, along with the masses of the unknown solute and BHT, to find the molar mass of the unknown solute.