MOLAR MASS BY FREEZING POINT DEPRESSION
advertisement

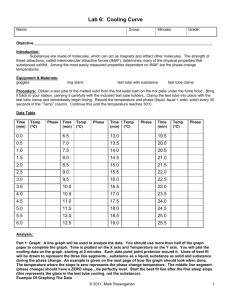
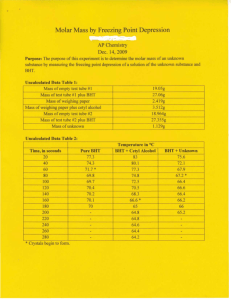
MOLAR MASS BY FREEZING POINT DEPRESSION The purpose of this experiment is to determine the molar mass of a substance by measuring its effect on the freezing point of a liquid. This effect is given by the equation Tf = Kf M ∆Tf is the change in freezing point, Kf is a constant for the solvent, and m is the solution molality. The method is to prepare samples of known mass composition and record the temperature during the phase change from liquid to solid for each sample (a cooling curve). The first sample is pure solvent (BHT, or butylated hydroxytoluene). The second sample is a solution of cetyl alcohol in BHT, from which you will determine the value of Kf for BHT. The third sample is a solution of unknown substance in BHT, from which you will determine the molar mass of the unknown. Procedure 1. Prepare three samples in three labeled test tubes: weigh the approximate amount of solid onto paper, tare a clean dry test tube, add the solid, and reweigh the tube to obtain the precise mass. If there are two solids in the tube, weigh each separately. tube 1 6-8 g BHT 2 6-8 g BHT + 1 g cetyl alcohol 3 6-8 g BHT + 1 g unknown 2. Prepare a boiling water bath in a 600 mL beaker. Next to the hot plate, set up a ring stand with utility clamp (for the tube) and 3-finger clamp (for the temperature probe). 3. Clamp tube #1 so that the solid in the tube will be completely surrounded by the bath. As the solid melts, put a copper stirring coil into the tube. When the solid is completely melted, raise it out of the water bath and clamp it off to one side, with the temperature probe well immersed in the liquid. Stir constantly and gently with the copper coil and record the temperature every 20 seconds until you have at least 6 points beyond the first appearance of crystals. 4. Remove the probe and copper coil and clean them thoroughly with warm ethanol and a tissue. Repeat step 3 using tube #2 and tube #3. As each solid mixture melts, stir it with the copper coil so that the mixture is homogeneous before you begin to record temperature readings. Analysis and Discussion 1. Plot temperature vs. time for each set of data. You can plot all three experiments on the same graph, using different colors. Determine Tf for each sample and record the values on the graph. 2. Calculate the molality of the solution in tube #2 (cetyl alcohol is C16H33OH, molar mass = 242.45 g/mol). Use the equation given in the introduction to determine the value of Kf for BHT. Remember, Kf describes a property of the solvent, so it doesn’t matter what the solute is. 3. Now that you know the value of Kf for BHT, use ∆Tf for tube #3 to determine the molality of the solution in tube #3. From the molality and the mass composition of the solution, determine the number of moles of solute present and the molar mass of the unknown. 4. The unknown is stearic acid (HC18H35O2, molar mass 284.5 g/mol). Calculate your percent error and discuss the reliability of your result.