File
advertisement

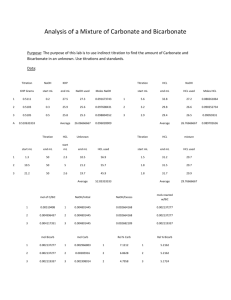
BACk-TITRATION A known accurately measured excess of a reagent is added to analyte . A portion of this added reagent reacts with the analyte and the unreacted portion (or the excess) is determined from the amount of titrant consumed. Addition reaction : aA + rR → pP Back-titration : rR + tT → nN Sample Problems 1. A 0.3207 g sample of primary standard grade benzoic acid, C6H5COOH, was dissolved in 50.00 ml solution of Ba(OH)2. The excess base required 4.20 ml of 0.1104 M HCl for back-titration. Calculate the concentration of Ba(OH)2 solution. 2. A 1.00mL aliquot of fish oil was analyzed for N using the Kjeldahl method. After digestion, the distilled ammonia was collected in 100.00 ml of 0.05030 M HCl. The excess HCl required 28.30 ml of 0.1240 M NaOH for titration. Calculate the amount of N in the sample as mg N/ml REDOX back-titration TITRATION 1. A 0.4586 g sample of pure iron was dissolved in acid, reduced to the divalent state and treated with 50.00 ml of KMnO4 solution. The excess KMnO4 was Back-titrated with 3.45 ml of 0.1200 M FeSO4. Calculate the M of the KMnO4 solution. 2. A 0.4353 g sample of primary standard grade Na2C2O4 was dissolved in acid and treated with 25.00 ml of 0.1200 M KMnO4. If the excess KMnO4 needed 15.60 ml of FeSO4 to back-titrate the excess KMnO4 What is the concentration of FeSO4 solution. 3. A 1.320 g sample of primary standard grade contains NaC2O4 was dissolved in acid, a portion of a 50.00 ml of 0.05063 KMnO4 was added to this solution. The excess KMnO4 was backtitratedwith 5.50 ml of 0.1760 M FeSO4 solution. Calculate the % of Na2C2O4 in the sample. 4. 150.0 mL of 0.2105 M nitric acid was added in excess to 1.3415 g calcium carbonate. The excess acid was back titrated with 0.1055 M sodium hydroxide. It required 75.5 mL of the base to reach the end point. 5. A 0.500g sample containing Na2CO3 is analyzed by adding 50.0ml of 0.100M HCL, a slight excess, boiling to remove CO2, and then back-titrating the excess acid with 0.100M NaOH . If 5.6ml NaOH is required for the back titration, what is the percent Na2CO3 in the sample? PRACTICE EXERCISES 1. A 0.5843 g sample of plant food preparation was analyzed for its N content by Kjeldahl method. The liberated NH3 was collected into a 50.00 ml solution of 0.05063 M HCl and 7.46 ml of 0.04917 M NaOH was needed to Back-titrate the excess HCl. Calculate the % (NH2)2CO in the sample. 2. A 5.00 L sample of urban air was bubbled through a solurion containing 50.00 ml of 0.01160 ml Ba(OH)2 which caused the CO2 to precipitate as BaCO3. The excess base was back-titrated with 23.60 ml of 0.01080 M HCl. Calculate the %( v/v) of CO2 in the air sample.The density of CO2 is 1.98 g/L 3. A 0.2750 g Sample of soda ash required 24.10 ml of 0.1684 M Hcl and a back-titration of 2.96 ml of 0.1005 M NaOH . Calculate the % Na2CO3 in the sample.