Acid Rain - CLSU Open University
advertisement
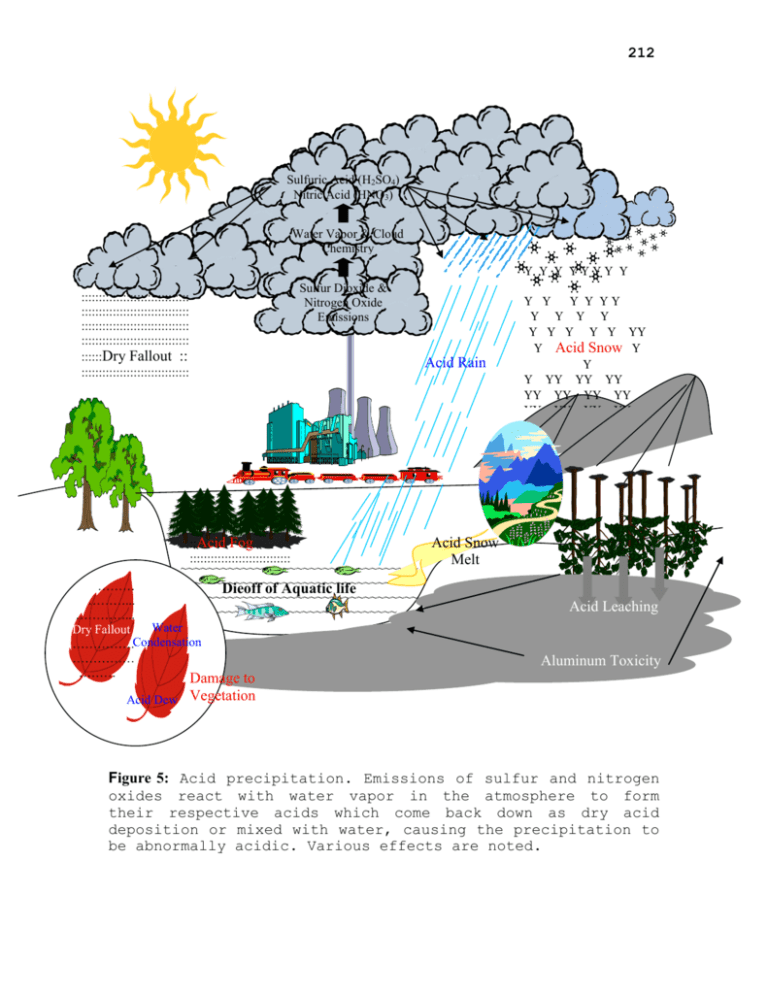
212 Sulfuric Acid (H2SO4) Nitric Acid (HNO3) Water Vapor & Cloud Chemistry Sulfur Dioxide & Nitrogen Oxide Emissions ::::::::::::::::::::::::::::::: ::::::::::::::::::::::::::::::: ::::::::::::::::::::::::::::::: ::::::::::::::::::::::::::::::: ::::::Dry Fallout :: ::::::::::::::::::::::::::::::: Acid Rain ::Acid Fog ::::::::::::::::::::::::::::: ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ ……… Dieoff of Aquatic life ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ ………... ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ …………... Water Dry Fallout ……………Condensation …………… ……… Damage to Acid Dew Acid Snow Acid Snow Melt Acid Leaching Aluminum Toxicity Vegetation Figure 5: Acid precipitation. Emissions of sulfur and nitrogen oxides react with water vapor in the atmosphere to form their respective acids which come back down as dry acid deposition or mixed with water, causing the precipitation to be abnormally acidic. Various effects are noted. 213 Atmospheric Turbidity One of the more obvious indications of atmospheric pollution is the presence of solid or liquid particles called aerosols dispersed in the air. These aerosols include dust, soot, salt crystals, organics, smoke, sulfates, ash, and a variety of microscopic particles. Collectively, they are often regarded as equivalent to air pollution although many of the materials involved are produced naturally by volcanic activity, forest and grass fires, evaporation, local atmospheric turbulence and biological processes (Fig. 6). The presence of aerosols provides a measure of atmospheric turbidity, a property which can be considered as an indication of dustiness or dirtiness of the atmosphere. Aerosols produced by human activities cannot match the volume of material produced naturally. STRATOSPHERE --------------------------------------TROPOSPHERE :::::::::::::::::::: ::::: soot:::::::: dust:::::::::::::: ::dust:: :::::::::: Forest fires Slash & burn ::::::::::::::::::: hydrocarbons: ::pollen::::::::: spores::::::::::: : H2O nitrates --------------- hydrocarbons Aviation ::::::::::::::::::: hydrocarbons: :::::::::::::::::: nitrates:::::::::: ::::sulphates::: ::: H2O ::: soot :::::::::dust:::: Soil particles Volcanic Activity dust Mining & Quarrying Natural organic emissions Urban & industrial activities Agriculture H2O :::::::::: Salt particles Ocean s Figure 6: Diagrammatic representation of the sources and types of atmospheric aerosols 214 Any increase in the turbidity of the atmosphere should cause global temperature to decline, as the proportion of solar radiation reaching the earth’s surface is reduced by scattering and absorption. In addition, the condensation of water vapor around atmospheric aerosols would lead to increased cloudiness and further reduction in the transmission of incoming radiation (Kemp, 1990). However, Mitchel (1975) proposed that although there is reduction in isolation, it is also considered that there would be a concomitant reduction in the amount of terrestrial radiation escaping into space, which would offset the cooling effect resulting in some warming of the lower atmosphere. Ozone Layer Depletion A layer of ozone gas in the upper atmosphere keeps the ultraviolet radiation within manageable limits. This ozone is a relatively minor constituent of the atmosphere. It is diffused through the stratosphere between 10 and 50 km above the surface reaching its maximum concentration at an altitude of 20-25 km. This small amount of a minor gas, with ability to filter out a very high proportion of the incoming UV radiation is essential for the survival of life on earth. Human activities has been found out to bring about sufficient degradation of the ozone layer of which recovery way never occur. These gases which destroy the ozone layer are the hydrogen oxides, nitrogen oxides, chlorine oxides and CFCs. The threat has been seen to come from four main sources- modern technological development in warfare, aviation, life-style and agriculture (Fig. 7). In addition, since UV radiation is an integral part of the earth’s energy budget, changes in the ultraviolet levels have the potential to contribute to climate change (Kemp, 1990; Cicerone, 1989). 215 Figure 7: Diagrammatic representation of the sources of natural and anthropogenic ozone-destroyers ENVIRONMENTAL IMPACTS OF CLIMATE CHANGE Physical Impact Mathematical models of the potential climate impact of a change in greenhouse gases concentrations have been developed by various groups (Rosenberg, 1989; Sinha, et al, 1989). The model attempt to predict changes in critical climate variable with a doubling of CO2 concentrations. While there is little agreement between various models about specific magnitudes of the changes at the regional level during the next 50-100 years, there is considerable agreement in the nature of such changes at the global level. 1. Atmosphere *Large Stratospheric Cooling A reduction in the upper stratosphere ozone by chlorine compounds means less absorption of solar radiation and thus less heating. This also means increased concentration of trace stratosphere. The combination of decreased heating and decreased cooling will decrease the upper stratospheric temperature perhaps by 10C to 20C (Silver & DeFries, 1990; Sinha et al, 1989).











