Exp Opt2 The Synthesis of Aspirin
advertisement

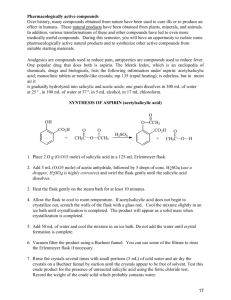
Experiment Optional #2: The Synthesis of Aspirin The natural world provides us with many of the medications in common use today. Taxol is the common name of a medication used in treating certain cancers; it is derived from the Yew tree. Most controlled substances, including heroin and cocaine, are extracted from plants. The deadly toxin associated with botulism, produced by bacteria, has even found a use in cosmetic surgery, although in a very diluted form. Salicylic acid, a compound found in willow bark, has long been known for its ability to reduce fever and suppress pain. Unfortunately, it is also tends to irritate and damage the upper digestive tract. A slight modification to its structure produces acetylsalicylic acid, which retains the benefits of the original compound with significantly less irritation. This popular medication goes by the common name Aspirin. Aspirin becomes salicylic acid in the lower digestive tract, where it is absorbed into the blood stream. O O O C OH O C + C H3C C O + CH3 acetic anhydride C HO O OH salicylic acid O OH C CH3 O aspirin (acetylsalicylic acid) acetic acid Procedure 1. Fill a large beaker with tap water. Place the beaker on a hot plate and heat the water inside to boiling. You should carry out steps 2 to 5 while it is heating. At some point you should also cool about 75 mL of DI water with ice for use in steps 8 and 11. 2. Weigh a 125-mL Erlenmeyer flask on the balance. Record this value on the report sheet. Remember, you should always write down all digits! 3. Add about 2 grams of salicylic acid to this flask, and reweigh. You can find the total mass of salicylic acid by subtraction. 4. In the hood, measure 5 mL of acetic anhydride into your small graduated cylinder. Then, slowly pour this into the Erlenmeyer flask from steps 2 and 3. 5. While still in the hood, add 10 drops of 85% phosphoric acid to this mixture (do not worry if you should accidentally add a few extra drops). Stir the mixture with a stirring rod. Page Optional #2-1 CH3 6. Place the flask in the hot water bath and stir the contents until all solids dissolve. Then, remove the flask from the bath and allow it to cool. 7. In the hood, slowly add 20 drops of DI water to the flask. 8. When the reaction is complete, pour in an additional 50 mL of ice-cold DI water. Then, place the flask in an ice bath (beaker with ice and some water) to cool for 10 minutes. 9. Slowly stir the contents. You should notice the formation of white, aspirin crystals. If not, gently scratch the inside of the flask with the glass stirring rod. This should induce crystals to grow. If this still does not work, ask your instructor to help you by seeding the mixture. 10. Setup a vacuum filtration according to your instructor’s directions. Attach the side arm of the filter flask to the water aspirator (or an alternative vacuum source if your instructor suggests one). Refer to Figure 12-1 for the setup (of course, you will not have any liquid in your filter flask when you begin!). ring stand Büchner funnel with wet filter paper covering holes utility clamp tubing attached to water aspirator Figure 12-1: Vacuum Filtration 11. Wash the crystals with enough ice-cold water to just completely cover the crystals. Allow the water to drain into the same flask you used in the previous step. 12. Carefully scoop the crystals onto your watch glass. These crystals are primarily composed of aspirin. Page Optional #2-2 Tests for Purity 1. Place five test tubes in a rack and label them from 1 to 5. 2. Add about 3 mL of the 0.15% salicylic acid solution to test tube 1. 3. Add a few crystals of each of the following to the other test tubes #2: crushed commercial aspirin #3: crushed buffered aspirin (if available) #4: the aspirin you prepared in the first procedure #5: acetylsalicylic acid 4. Add 5 drops of 1% ferric chloride (FeCl3) solution to each test tube. A purple color indicates that there is salicylic acid present. The more acid there is in the test tube, the deeper the color. 5. Write down your observations on the report sheet. Page Optional #2-3 Page Optional #2-4 The Synthesis of Aspirin Report Sheet Name:________________________ Mass of Erlenmeyer Flask: ____________g Mass of Erlenmeyer Flask and Salicylic Acid:______________g Mass of Salicylic Acid:________________g Describe the appearance of the aspirin you have prepared. Did you notice any kind of smell on your aspirin (before filtering)? If so, where did this smell probably come from? Describe the appearance of test tubes 1-5 after adding FeCl3: #1: #2: #3: #4: #5: Page Optional #2-5 Commercial aspirin is not allowed to contain more than 0.15% salicylic acid. Compare test tubes 2-5 with test tube 1. Which of these appear to contain more than 0.15% salicylic acid? If you had a headache right now, would it be a good idea to ingest the aspirin you prepared? Why or why not? Which functional group is present in salicylic acid that is not present in aspirin? Circle and identify all functional groups in the common analgesics below. O C HO N CH3 CH3 H2C H C H3C CH CH3 acetaminophen (active ingredient in Tylenol) CH HO ibuprofen (active ingredient in Advil & Motrin) Page Optional #2-6 O Lab Preparation for Experiment 12: Synthesis of Aspirin ice salicylic acid (3 containers, 25-50 grams per container) 85% phosphoric acid (2-3 small dropper bottles) in hood acetic anhydride (at least 2 bottles, no droppers, 100 mL) in hood 1% FeCl3 aqueous solution (at least 3 small dropper bottles) 0.15% salicylic acid aqueous solution (3 bottles, 50+ mL per bottle, droppers okay) Please be sure that the concentration of solution is accurate to at least 2 significant digits 1 vacuum filtration assembly per student, including filter paper 6-8 mortar and pestles Labeled samples of each of these: commercial aspirin buffered aspirin (examples: Ascriptin, Bufferin, Cama, Easprin, Ecotrin, Empirin) Bufferin and Ecotrin should be easy to get. acetylsalicylic acid (3 containers, 25-50 grams per container) organic waste container iron waste container (if necessary) Page Optional #2-7