putis_usman_chap2_reviewed
advertisement

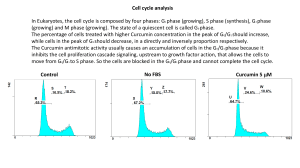
Chapter 2 Review of Related Literature Curcumin The expression of various cell surface adhesion molecules such as intercellular cell adhesion molecule-1, vascular cell adhesion molecule-1, and endothelial leukocyte adhesion molecule-1 on endothelial cells is absolutely critical for tumor metastasis (Ohene-Abuakwa and Pignatelli, 2000). The expression of these molecules is in part regulated by NF-κB (Iademarco et al., 1995). We have shown that the treatment of endothelial cells with curcumin blocks the cell surface expression of adhesion molecules, and this accompanies the suppression of tumor cell adhesion to endothelial cells (Kumar et al., 1998). We have demonstrated that downregulation of these adhesion molecules is mediated through the downregulation of NF-κB activation (Kumar et al., 1998). Gupta and Ghosh reported that curcumin inhibits TNF-induced expression of adhesion molecules on human umbilical vein endothelial cells (HUVECs). (Jaiswal et al. 2002) showed that curcumin treatment causes p53- and p21-independent G(2)/M phase arrest and apoptosis in colon cancer cell lines. Their results suggest that curcumin treatment impairs both Wnt signaling and cell–cell adhesion pathways, resulting in G(2)/M phase arrest and apoptosis in HCT-116 cells. (Aggarwal et al., 2006) The presence of the diketone moiety in the curcumin molecule seems to be essential in breaking down the molecules of organic matter. (Hanne Hjorth Tc–nnesen and John V. Greenhill , 1992) Turmeric Study of MEM. Braga et. al, 2003 showed 60% of turmeric extracts were composed of ar-turmerone, (Z)-y-atlantone and (E)-y-atlantone extracted through different techniques hydrodistillation, low pressure solvent extraction, Soxhlet, and supercritical extraction using carbon dioxide and cosolvents. The solvents and cosolvents tested were ethanol, isopropyl alcohol, and their mixture in equal proportions. The composition of the extracts was determined by gas chromatography−flame ionization detection (GC-FID) and UV. For the supercritical extraction, the best cosolvent was a mixture of ethanol and isopropyl alcohol. except for the Soxhlet extracts (1:100, ethanol), for which only ar-turmeronol and (Z)-α-atlantone were detected. The maximum amount of curcuminoids (8.43%) was obtained using Soxhlet extraction (ethanol/isopropyl alcohol). The Soxhlet and low pressure extract exhibited the strongest antioxidant activities. Ethyl Alcohol to Trap Curcumin Curcumin is extracted from the dried root of the rhizome Curcuma Longa. The process of extraction requires the raw material to be ground into powder, and washed with a suitable solvent that selectively extracts colouring matter. This process after distillation of the solvent yields an oleoresin with colouring matter content in the region of 25-35 percent along with volatile oils and other resinous extractives. The oleoresin so obtained is subjected to further washes using selective solvents that can extract the curcumin pigment from the oleoresin. This process yields a powdered, purified food colour, known as curcumin powder, with over 90 percent colouring matter content and very little volatile oil and other dry matter of natural origin. The selection of solvents is done with care to meet extractability and regulatory criteria. The following solvents are considered suitable: Isopropanol In the curcumin manufacturing process isopropyl alcohol is used as a processing aid for purifying curcumin. Ethyl acetate With a restriction placed on the use of chlorinated solvents, such as dichloroethane, it is found that ethyl acetate, owing to its polarity, is a reasonable replacement providing acceptable quality of product and commercially viable yields. Acetone This is used as a solvent in the curcumin manufacturing process. Carbon dioxide This is not currently used in commercial production. However, it is listed in EC Directive 95/45/EC and has potential as a substitute for chlorinated solvents. Methanol This solvent is used occasionally as a processing aid for purification. Ethanol This solvent is used sparingly because curcumin is completely soluble in ethanol. (Ivan Stankovic, 2004) Decomposition Study of GH Bouder,1940 relates to a process of apparatus for stabilizing and digesting decomposable organic matter, more particularly domestic and industrial organic wastes, with the aid of the thermophilie micro-organisms, already in the material, and to the product obtained thereby. An object of the invention is to convert such decomposable organic matter into a product having valuable properties. It is also an object to produce such a product which may be employed in treatment of other undecomposed organic matter and one which may be useful as a fertilizing material. Another object is the production of improved products having valuable properties for use as a filter aid, a filtering material, or as a purifying agent as well as for various other uses. A further object of the invention is to treat waste organic matter in such a manner that it is thoroughly decomposed while inhibiting putrefaction and microorganic fermentation. Water purification experiments to decompose phenol, acetic acid, and Rhodamine B in water were conducted using a direct contact of gas corona discharge to the water surface. It was shown that O2 was important in the gas phase for the degradation process, and the negative corona showed higher degradation rates than the positive corona. It was found that the organic contaminants can effectively be decomposed by the present method without pH adjustment. The experimental results indicated that there were optimized values in the O2 concentration, the gas resident time above the water, and the cathode−anode gap. It was also indicated that the O2/CO2 mixture showed a higher degradation rate than the O2/N2 mixture for the gas phase. As the degradation mechanism, the uncharged short-lived radicals are considered to be important. (N Sano 2002) This review deals with methodological approaches, measured rates, and environmental control of two major interdependent processes regulating the structure and function of terrestrial ecosystems, viz., plant decomposition and soil respiration. Both these processes have been evaluated through indirect assessments as well as through direct measurements under the field conditions. The techniques used suffer in general from difficulties in creating conditions of natural environment during the process of measurement. Generalizations regarding the magnitude of rates in different ecosystems are difficult because of limited results or non-comparability of results from different methods. Temperature and moisture and their interactions markedly influence both the processes. The surface feeders and soil animals have a marked influence on the decomposition. Partitioning of soil respiration into components due to live roots, microbes, and soil fauna has eluded a satisfactory solution so far. Banana Peels Banana peels take about three to four weeks to compost, according to the Missouri Department of Conservation. It is better to hot compost them outdoors than to cold compost them indoors in a worm bin. Cutting the peels into small pieces before adding them to a compost heap speeds the process.