Topic 4, Atoms, Elements and Compounds
advertisement

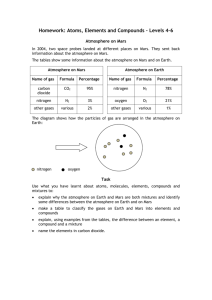
Homework: Atoms, Elements and Compounds – Levels 6–8 Atmosphere on Mars In 2004, two space probes landed at different places on Mars. They sent back information about the atmosphere on Mars. The tables show some information about the atmosphere on Mars and on Earth. Atmosphere on Mars Atmosphere on Earth Name of gas Formula Percentage Name of gas Formula Percentage carbon dioxide CO2 95% nitrogen N2 78% nitrogen N2 3% oxygen O2 21% other gases various 2% other gases various 1% The diagram shows how the particles of gas are arranged in the atmosphere on Earth: nitrogen oxygen Task Use what you have learnt about atoms, molecules, elements, compounds and mixtures to: identify some differences between the atmosphere on Earth and on Mars explain, using examples from the tables, the differences between an element, a compound and a mixture and the difference between an atom and a molecule make a table to show the names and numbers of atoms in the molecules of the gases in the table give reasons why the diagram of the particles in air is a good model but is not a true representation of particles in real air. Mark Scheme Level You should: 6 explain the meaning of ‘compound’ in terms of atoms explain how compounds are different to mixtures identify at least three differences between the atmosphere on Earth and on Mars by talking about both the types of gases and the percentages. 7 explain the difference between an atom and a molecule draw a table to show the names of each element in the gases on Mars and on Earth with the number of atoms in each molecule of gas. 8 describe at least one reason why the diagram of particles in air is a good model, for example ratio of nitrogen to oxygen, particles are arranged as in a gas, mixture of particles shown identify at least two reasons why the model of air is different to real air using ideas about size or closeness of particles, missing gases or molecules shown as single atoms.