Quiz Study Guide Compounds, States of Matter, and Phase
advertisement

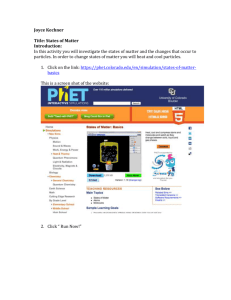
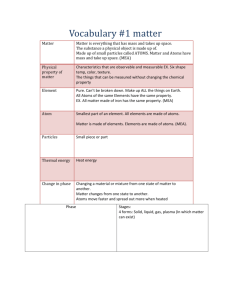
Quiz Study Guide Compounds, States of Matter, and Phase Changes Tuesday November 26th Multiple choice questions will cover the following topics: 1. Compounds- reading the formula. Can you tell us what elements and how many atoms of each element are found in common compounds? Example: H2O- contains elements hydrogen and oxygen. 2 atoms of hydrogen, 1 atom of oxygen, 3 atoms total in this molecule. 2. Three states of matter- how are the particles moving (fast, slow, close together, far apart) for the three states of matter? What shape and volume do each state of matter have? Example: Particles in a solid are close together and vibrate slowly 3. Phase Changes- What do we call each phase change? Look at your phase change pyramid. How do we get matter to change phases? What do the particles/molecules look like as they change phases? (Do the elements separate/stay together? Do the molecules move closer together and slow down or do they spread apart and speed up?) Example: Solid to a gas is called ___________ (sublimation) energy is being ______ (added) Quiz Study Guide Compounds, States of Matter, and Phase Changes Tuesday November 26th Multiple choice questions will cover the following topics: 4. Compounds- reading the formula. Can you tell us what elements and how many atoms of each element are found in common compounds? Example: H2O- contains elements hydrogen and oxygen. 2 atoms of hydrogen, 1 atom of oxygen, 3 atoms total in this molecule. 5. Three states of matter- how are the particles moving (fast, slow, close together, far apart) for the three states of matter? What shape and volume do each state of matter have? Example: Particles in a solid are close together and vibrate slowly 6. Phase Changes- What do we call each phase change? Look at your phase change pyramid. How do we get matter to change phases? What do the particles/molecules look like as they change phases? (Do the elements separate/stay together? Do the molecules move closer together and slow down or do they spread apart and speed up?) Example: Solid to a gas is called ___________ (sublimation) energy is being ______ (added)