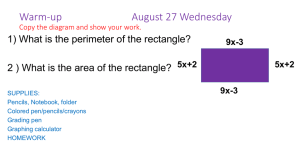
Probability
advertisement

6.3 Diffusion and Brownian Motion.
Brownian motion is a probablistic model of certain types of motion. For example
1.
Diffusion of particles in gases, e.g. diffusion of water molecules in air
2.
Diffusion of particles in liquids, e.g. diffusion of sugar molecules in water
3.
Heat conduction
4.
Movement of stock prices
Brownian motion is a continuous version of a random walk which is a discrete model of certain types of
motion which have a random aspect. So, we first take a look at random walks and then see how we get
Brownian motion as the limit of random walks.
Random Walks. A random walk is a special type of Markov chain in which one gets from one state to
another by adding a random amount. As an example we shall consider the motion of a water molecule in
air. For simplicity we shall assume its motion is in one dimension which we take as the x axis.
Water molecules move fairly fast at room temperature. A typical speed is
v = 100 m/sec 0.06 miles/sec 200 miles/hr
It doesn't go very far until it hits a nitrogen or oxygen molecule in the air. A typical distance before it hits
another molecule is
l = 5 10-5 cm = mean free path = average distance between collisions
Let's suppose when it hits it rebounds in the opposite direction with the same speed v. Let
l
x = 2 = 2.5 10-5 cm = ½ average distance between collisions
We assume that after the water molecule has gone a distance x it has a 50% chance of striking another
molecule in the air. If it does it rebounds and goes in the opposite direction with velocity v. If it doesn't,
then the water molecule continues in the same direction with velocity v and goes another distance x and
then again has a 50% chance of a collision. So on the average it goes a distance 2(x) = l before it strikes
another molecule. Let
t =
x
v
=
l
= 2.5 10-9 sec = time it takes to go a distance x
2v
We construct a Markov chain model of the water molcule's motion as follows. The states are the points on
the x axis whose coordinates are multiple of x, i.e the points j(x) where j is an integer, positive, negative
6.3.1 - 1
or zero. We consider the position of the water molecule at times which are non-negative integer multiples
of t, i.e. n(t) where n is a positive integer of zero. Let
Un = displacement of water molecule during the time interval (n – 1)(t) < t < n(t)
We treat Un as a random variable and assume that there is a 50% chance the velocity during the time
interval (n – 1)(t) < t < n(t) is either v or – v. So Pr(Un = x) = ½ and Pr(Un = - x) = ½. The Un all
have the same distribution. We also assume that the Un are all independent of each other. Note that
E{Un} = mean of Un = 0
E{(Un)2} = (1/2)(- x – 0)2 + (1/2)(- x – 0)2 = (x)2
Un = standard deviation of Un = x
We let
X(t) = the position of the water molecule at time t
Yn = X(n(t)) = the position of the water molecule at time n(t)
In particular,
X(t) = Yt/(t)
Y0 = X(0) = starting position of the water molecule at time t = 0
Y1 = X(t) = the position of the water molecule at time t
Y2 = X(2(t)) = the position of the water molecule at time 2(t), etc
One has
Yn = Yn-1 + Un
i.e. the state at time n is obtained by adding Un to the state at time n – 1. It follows that
Y1 = Y0 + U1
Y2 = Y0 + U1 + U2
Yn = Y0 + U1 + U2 + + Un = Y0 + Vn
where
Vn = U1 + U2 + + Un = Yn - Y0 = X(n(t)) - X(0)
6.3.1 - 2
or
X(t) - X(0) = Vt/(t)
If n = t/(t) is large, then by the central limit theorem Vn is approximately normal with mean 0 and standard
deviation
n (Un) =
n (x) =
t
t
(x) =
t
where
(x)2
=
t
=
vl
2
For the water molecule this works out to be
(104 cm/sec)(5 10-5 cm)
=
2
=
0.25 cm2/sec = 0.5 cm/(sec)1/2
So at time t the water molecule's displacement X(t) - X(0) is approximately normal with mean zero and
vl
standard deviation t with =
. So the density function of X(t) - X(0) is approximately
2
1
2
2
e-x /(2 t) =
22t
ft(x) =
1
2
e-x /(4Dt)
4Dt
where
D =
2
2
=
vl
= Diffusion coefficient
4
Note that
=
2D
For example, if t = 25 sec and = ½ cm/(sec)1/2 then f25(x) =
1
2
2
e-x /(2 t) =
22t
1
2
e-x /12.5.
2 (2.5)
and D are macroscopic parameters that describe the motion of the water molecules or whatever particles
one is modeling by diffusion. Most science and engineering books tend to use the diffusion coefficient D
instead of . However, sometimes is sometimes more convenient for hand computations using tables of
the normal distribution. For the water molecule the diffusion coefficient is
D =
2
2
=
(0.5 cm/(sec)1/2)2
= 0.125 cm2/sec
2
6.3.1 - 3
Another way of saying that the displacement X(t) - X(0) at t is approximately normal with mean zero and
standard deviation
t is to say that Z(t) = (X(t) – X(0)/( t) is approximately a standard normal random
variable.
Example 1. What is the approximate probability that a water molecule is a distance of at least 3 cm from
its starting point after 25 sec. Assume = 0.5 cm/sec1/2.
We want Pr{ | X(25) – X(0) | > 3}. In this case Z = (X(25) – X(0)/((0.5) 25) = (X(25) - X(0)/2.5 is
approximately a standard normal random variable. So
Pr{ | X(25) – X(0) | > 3} = Pr{ |
X(25) – X(0)
| > 3/(2.5)} = Pr{ | Z | > 1.2} = 2 Pr{ Z > 1.2}
2.5
= 2(1 - Pr{ Z 1.2}) = 2(1 - (1.2)) = 2(1 – 0.8849) = (2)(0.1151) = 0.2302
Here
z
(z) = Pr{Z z} =
1
2
e-u /2 du
2
-
is the cummulative distribution function of a standard normal random variable. It is available in
mathematics software and tables are usually included in statistics books.
If we are given that X(0) = then X(t) is normal with mean and standard deviation t, so its density is
1
2
2
pt(x) =
e-(x - ) /(2 t).
22t
Brownian Motion. If we look at the motion of the water molecule in the limit that the mean free path l
vl
goes to zero and the velocity v goes to infinity in such a way that =
stays constant the resulting
2
motion is Brownian motion X(t). It has the following properties
1.
X(s + t) – X(s) is normal with mean zero and standard deviation t
2.
If 0 < t0 < t1 < < tn then the displacements
X(t1) – X(t0)
X(t2) – X(t1)
4
…
X(tn) – X(tn-1)
3
are all independent
2
Above we focused on the motion of a single water
1
molecule. Now suppose we have a bunch of water
molecules. In particular, suppose we are given an initial
4
6.3.1 - 4
2
2
4
density c0(x) of water molecules in the air. For example, we might have
(1)
4 10-3 gm/cm
c0(x) =
0
if | x | < 2
otherwise
We would like to find the density ct(x) of water molecules at a later time t. The initial density c0(x) acts like
a probability distribution. If we let A =
c0(x) dx the p0(x) = c0(x)/A is the initial probablilty density
-
function of X(0). Note that X(t) = X(0) + (X(t) – X(0)). X(0) and X(t) – X(0) are independent, so to get the
probablity density function of X(t) we convolute the density function c0(x)/A of X(t) with the density
1
2
2
function
e-x /(2 t) of X(t) – X(0). Finally we multiply by A to get the density ct(x) of water
22t
molecules. Thus
1
e-(x - )2/(22t) c0() d
22t
ct(x) =
-
Using the formula (1) for c0(x) we have
2
ct(x) = (4 10 )
-3
1
e-(x - )2/(22t) d
22t
-2
If we substitute z = (x - )/(
t) the integral becomes
1
ct(x) = (4 10 )
2
-3
(x-2)/( t)
(x+2)/( t)
1
-z2/2
-3
e (-1) dz = (4 10 ) 2
(x+2)/( t)
x + 2
x - 2
= (4 10-3) [
-
]
t
t
Suppose t = 25. Then
-z2/2
e dz
(x-2)/( t)
4
3
t = (1/2)(5) = 2.5 cm so
2
x + 2
x - 2
ct(x) = (4 10-3) [
2.5 - 2.5 ]
A graph is at the right.
1
10
5
5
Problem 1. The diffusion coefficient of (ethyl) alcohol in air is D = 0.1 cm2/sec. Consider the
diffusion of alcohol in one dimension.
a. Suppose an alcohol molecule starts at position x = 2 cm. What is the probability that its
position 10 seconds later will be greater than or equal to x = 2.5 cm?
b. Suppose the density of alcohol to begin with is given by
c0(x) =
0
x
1
for x < 0
for 0 x 1
for 1 < x
6.3.1 - 5
10
Find a formula for the density ct(x) of ammonia at time t.
Solution to a. We want Pr{ X(10) – X(0) > 0.5}. 2DIn this case t = 2D t = 2Dt =
(2)(0.1)(10) = 2. We know that X(10) – X(0) is normal with mean 0 and standard deviation
t. So Z = (X(10) – X(0))/ 2 is approximately a standard normal random variable. So
X(10) – X(0)
Pr{ X(10) – X(0) > 0.5} = Pr{
| > 0.5/ 2} = Pr{Z > 2/4}
2
= 1 - Pr{Z
2/4}) = 1 - ( 2/4)) 1 – (0.35)) 1 – 0.6368 = 0.3632
Brownian Motion with Drift. Let's look again at water molecules diffusing in air. So far we have been
assuming that the air as a whole has no net motion. However, it may happen that the air as a whole is
moving with a certain net velocity. Let
= net velocity of the air
= drift rate
Then water molecules inherit this drift and the diffusion is superimposed on top of this drift. The result is
that at time t the water molecule's displacement X(t) - X(0) is now approximately normal with mean t and
standard deviation
t. So the density function of X(t) - X(0) is approximately
ft(x) =
1
2
2
e-(x - t) /(2 t)
22t
Example 2. Suppose a water molecule diffuses in air with = 0.5 cm/sec1/2. Suppose also the air has a
drift of = 2 cm/sec. What is the probability that a water molecule will have traveled a distance of between
30 and 34 cm in the positive direction after 16 sec.
We want Pr{ 30 < X(16) – X(0) < 34}. In this case t = (2 cm/sec)(16 sec) = 32 cm and
t = (0.5 cm/sec1/2)(4 sec1/2) = 2 cm. We know that X(16) – X(0) is normal with mean t and standard
deviation t. So Z = (X(16) – X(0) – 32)/2 is approximately a standard normal random variable. So
Pr{ 30 < X(16) – X(0) < 34} = Pr{ - 2 < X(16) – X(0) – 32 < 2} = Pr{ -1 <
X(16) – X(0) - 32
< 1}
2
= Pr{- 1 < Z < 1} = 2 Pr{0 < Z < 1} = 2(Pr{Z < 1} – ½) = 2((1) – ½)
= 2(0.8413 – 0.5) = (2)(0.3413) = 0.6826 68%
Problem 2. Redo problem 1 if the air is drifting in the negative x direction at a rate of 0.05
cm/sec.
6.3.1 - 6
Appendix. Here are the values of some diffusion constants, D.
Diffusion in air:
Hydrogen, H2
Water vapor, H2O
Carbon monoxide, CO
Ammonia, NH3
Methane, CH4
Oxygen, O2
Nitrogen, N2
Hydrogen cyanide
Carbon dioxide, CO2
Mercury
Ethyl alcohol
Ethyl ether
1.3
0.22
0.21
0.2
0.2
0.18
0.18
0.17
0.16
0.12
0.1
0.079
cm2 / secanother source gave 0.74
cm2 / sec
cm2 / sec
cm2 / sec
cm2 / sec
cm2 / sec
cm2 / sec
cm2 / sec
cm2 / sec
cm2 / sec
cm2 / sec
cm2 / sec
Diffusion in water:
Alcohol
Caffein
Sugar
Dyes
Proteins
10
6
6
2-6
0.4 - 1
10-6 cm2 / sec
10-6 cm2 / sec
10-6 cm2 / sec
10-6 cm2 / sec
10-6 cm2 / sec
6.3.1 - 7