Amount of Fat in Potato Chips
advertisement

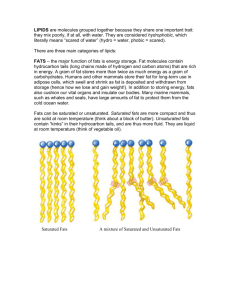
Experiment-13: Amount of Fat in Potato Chips Chemistry of Lipids Quantitative determination of Fat Potato Chips DO NOT USE BUNSEN BURNER! NO FLAMES IN THIS EXPERIMENT!! The typical Standard American Diet is becoming worse, possibly due to the fast paced lives and easy accessibility to fast food restaurants. Obesity and Diabetes are on the rise, Heart Disease is still the number one killer in the United States. There is much more awareness of Calories count etc., yet average an American gets approximately 40% Calories from Fats. Nutritionists recommend that no more than 30% of our daily 2000 Calories come from fats. Which, of course, is not surprising at all when you consider that the per capita consumption of potato chips in the US is 16.9 lbs. Not all fat is bad - on the contrary, some fats such as omega-3 fatty acids are essential, highly nutritious and beneficial for weight control. Carbohydrates, proteins and Fats are three major groups, which supply energy to our bodies. Although fat may not be the preferred fuel of the body, it does supply more energy per gram than any other nutrient: Carbohydrates: 4 Cal/g, Protein: 4 Cal/g and Fat = 9 Cal/g. Lipids are compounds of fatty acids and glycerol. Lipids are the most efficient source of fuel in living things; they are stored beneath the skin in animals and the human, and mostly in the seeds of plants. Food lipids are divided into fats, which come from animal sources and are solid at room temperature, and oils, which come from plant sources and are liquid at room temperature. Another type of lipid is cholesterol. Cholesterol is a sterol compound made by animals and is used to make certain steroid hormones in the body. It is not found in plants. A fatty acid is a carboxylic acid derived from or contained in an animal fat or vegetable oil. All fatty acids are composed of alkyl groups or hydrocarbon chains containing from 4 to 22 carbon atoms and characterized by a terminal carboxyl group COOH. Fatty acids are the building blocks of fats, having hydrogen atoms attached to chains of carbon atoms. Fatty acids are found in every cell of the human body. Fats are insoluble in water (a fat is non-polar, and water is very polar). Fats are soluble in nonpolar solvents such as petroleum ether. Lipase is a generic name given to a group of enzymes that catalyze the hydrolysis of lipids. For example, a lipase that works on food lipids breaks down triacylglycerol into glycerol and fatty acids. A triacylglycerol is a lipid compound consisting of three fatty acids linked to one glycerol molecule. This compound is an important source of energy for the human body. To utilize this compound for energy, enzymes called lipases must first hydrolyze it to liberate the fatty acids that are chemically bonded to glycerol. Lipids are classified as organic compounds that are soluble (dissolvable) in organic solvents, but only sparingly soluble in water. Lipids are biologically important for making barriers (membranes of animal cells), which control the flow of water and other materials into a cell. Lipids include fats, oils, waxes, cholesterol, other sterols, and most steroids. In the body, fat serves as a source of energy, a thermal insulator and cushion around organs, and an important cellular component. The fat-soluble vitamins are A, D, E, and K. Since fats have 2.25 times the energy content of carbohydrates and proteins, most people try to limit their intake of dietary fat to avoid becoming overweight. The food industry has a big market for low-fat and non-fat foods. Fats and oils make up 95% of food lipids and phospholipids, and sterols make up the other 5%. Traditionally, fats were considered to be solid at room temperature, and oils were considered to be liquid. However, this designation is often used to distinguish between fats and oils from animals and plants, respectively. Saturated fats: The molecules contain only single bonds between ant two carbon atoms. No double or triple bonds. Saturated fat is the main dietary cause of high blood cholesterol. The American Heart Association recommends the intake of saturated fat to 7–10 percent of total calories (or less) each day. Saturated fat is found mostly in foods from animals and some plants. Foods from animals — these include beef, beef fat, veal, lamb, pork, lard, poultry fat, butter, cream, milk, cheeses and other dairy products made from whole milk. These foods also contain dietary cholesterol. Foods from plants — these include coconut oil, palm oil and palm kernel oil, and cocoa butter. Hydrogenated fats: During food processing, fats may undergo a chemical process called hydrogenation. This is common in margarine and shortening. These fats also raise blood cholesterol. Use hydrogenated fats only if they contain no more than two grams of saturated fat per tablespoon. The saturated fat content of most margarines and spreads is printed on the package or Nutrition Facts label. Polyunsaturated and monounsaturated fats — Polyunsaturated and monounsaturated fats are the two unsaturated fats. They're found primarily in oils from plants. Polyunsaturated fats — These include safflower, sesame and sunflower seeds, corn and soybeans, many nuts and seeds, and their oils. Monounsaturated fats — These include canola, olive and peanut oils, and avocados. Both polyunsaturated and monounsaturated fats may help lower your blood cholesterol level when you use them in place of saturated fats in your diet. But a moderate intake of all types of fat is best. Use polyunsaturated or monounsaturated oils — and margarines and spreads made from them — in limited amounts. This is recommended in place of using fats with a high saturated fat content, such as butter, lard or hydrogenated shortenings. Trans-fatty acids: Unsaturated fatty acids can be in one of two shapes — "cis" and "trans." These terms refer to the physical positioning of hydrogen atoms around the carbon-carbon double bonds in the carbon chain. The cis form is more common than the trans form. Trans-fatty acids (TFA) are found in small amounts in various animal products such as beef, pork, lamb and the butterfat in butter and milk. TFA are also formed during the process of hydrogenation, making margarine, shortening, cooking oils and the foods made from them a major source of TFA in the American diet. Partially hydrogenated vegetable oils provide about three-fourths of the TFA in the U.S. diet. In clinical studies, TFA or hydrogenated fats tend to raise total blood cholesterol levels. Some scientists believe they raise cholesterol levels more than saturated fats. TFA also tend to raise LDL ("bad") cholesterol and lower HDL ("good") cholesterol. These changes may increase the risk of heart disease. The FDA requires trans fat to be listed on the nutrition label. Food manufacturers have until 2006 to comply. Many fast foods contain high levels of TFA. There are no labeling regulations for fast food, and it can even be advertised as cholesterol-free and cooked in vegetable oil. Eating one doughnut at breakfast (3.2 g of TFA) and a large order of French Fries at lunch (6.8 g of TFA) can add 10 g of TFA to one's diet, so the lack of regulations for labeling restaurant foods can be harmful to health. Procedure: DO NOT USE BUNSEN BURNER! NO FLAMES IN THIS EXPERIMENT!! DO NOT TASTE ANY FOOD ITEMS IN THIS LABORATORY !!! 1. Find the mass of a dry and clean 125-mL Erlenmeyer flask. Record all the digits throughout the experiment. Use the same scale throughout this experiment. Put approximately 10 to 15 g of crushed potato chips into this Erlenmeyer flask. Now, find the mass of the 125-mL Erlenmeyer flask with potato chips. 2. Find the mass of a dry and clean 150-mL or 100-mL beaker. 3. Using a graduated cylinder, transfer 20 mL petroleum ether into the 125-mL Erlenmeyer flask containing potato chips. Swirl the contents carefully for few minutes. At this stage, you are extracting lipid (oil) from the potato chips by swirling and mixing. You will notice the color of petroleum ether changes to light yellow from colorless. 4. Carefully decant the top yellow liquid (petroleum ether + lipid) into the preweighed beaker from step-2. Do not transfer any chips. 5. Using a graduated cylinder, transfer another 20 mL portion of petroleum ether into the 125-mL Erlenmeyer flask. Swirl the contents carefully for few minutes. At this stage, you are trying to extract last traces of lipid still present in potato chips. 6. Carefully decant the top yellow liquid into the same beaker. Do not transfer any chips. Now, this beaker contains a yellow liquid, which is approximately 40 mL (20 + 20) petroleum ether plus lipid extracted from the potato chips. 7. Electrical hot plates have been placed inside the hoods. Use only low settings. Do not bring these hot plates to your bench. Put your beaker from step-6 on top of the hot plate. If few minutes you will observe bubble formation. This is due to the evaporation of petroleum ether, which is very volatile and highly flammable. Continue the heating process until you see no more bubbling. You will notice that the lipid, a thick yellow liquid, is left over in the beaker. Shut-off and unplug the hot plate. Remove the beaker and cool it. Find the mass of beaker with lipid. 8. Calculate % lipid = (mass of lipid extracted / mass of chips) x 100 Laboratory Report#13: Amount of Fat in Potato Chips Last Name_____________________________, first name________________ Date of Experiment______________ Instructor’s Initials__________ Data and Calculations 1. Mass of a dry and clean 125-mL Erlenmeyer flask =_____________________g 2. Mass of the 125-mL Erlenmeyer flask plus potato chips =_____________________g 3. Mass of potato chips = #2 - #1 =_____________________g 4. Mass of dry and clean 150-mL beaker =_____________________g 5. Mass of the 150-mL beaker plus lipid extracted =_____________________g 6. Mass of lipid extracted = #5 - #4 =_____________________g 7. % of lipid in potato chips = (mass of lipid extracted / mass of chips) x 100 = (#6 / #3) x 100 = _____________% = Experimental value 8. Calculate the % of lipid in potato chips from Nutritional label = (grams of fat per serving/ serving size in grams) x 100 = _____________% = Theoretical value 9. Calculate the % error = (Difference between theoretical and experimental values/ theoretical value) x 100 = (Difference between step-7 and step-8÷step-8) x 100=_______% Show all your work! Use proper units and significant figures!! 1. How many grams of fat does a 5.5 oz bag of potato chips provide? (Read Label) 2. What is Olestra? (Do Internet research) 3. How many grams of Protein does a normal adult require everyday? 4. What is an Iodine Number of a Lipid? (Do Internet research)