l3 - Arizona State University
advertisement

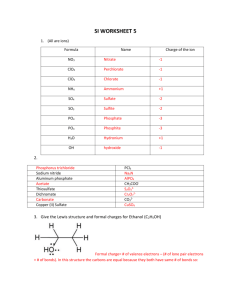
Copyright, Arizona State University CHM 233 Review Resonance 1 Electron Delocalization Q. Which of the following two Lewis structures is correct? H H resonance brackets O C resonance arrow H N H O C H O = N H C O 1/2 N O 1/2 H "actual" structure two resonance CONTRIBUTORS resonance hybrid A. Neither are wrong but neither give a proper description of where the electrons are in the molecule, a proper description of CH3NO2 requires a CONTRIBUTION from BOTH LEWIS STRUCTURES H O H Example: Allyl Cation • Each carbon of the allyl cation (below) is sp2 hybridized • Add the p A.O.s that make the pi-bond and the "empty" p A.O. on the carbon with the positive charge • Which 2 p A.O.s make the pi bond and which is empty? the molecule isn't sure either!! these 2 make the p-bond two resonance CONTRIBUTORS H 50% C H + H C C H H 50% H H C C H why don't these 2 make a p-bond? they do!! H C H = H resonance arrow 1/2 H C C C H 1/2 H H "actual" structure resonance hybrid resonance brackets • Neither of these two Lewis structures are, on their own, correct. The correct (actual) structure is a RESONANCE MIXTURE or HYBRID of the two Lewis structures, they are both CONTRIBUTE via RESONANCE RESONANCE, when MIXED (hybridized) together properly describe the overall structure • resonance contributors are NOT isomers, they represent the REQUIRED different ways of indicating where delocalized electrons are within the "confines" of the Lewis structure "language", they are different ways of drawing the SAME THING! Example: Allyl Anion EACH CONTRIBUTES 50% H H these 2 make the pbond H C C H C H C H H these 2 MUST also make a p-bond H H C C H H H sp2 C sp2 H = 1/2 C C 1/2 H H "actual" structure resonance hybrid • ALL THREE carbons must be sp2 hybridized to allow formation of both pi-bonds • "actual" hybrid (mixed) structure has all electrons that can be involved in bonding in partial (dashed) bonds, so no non-bonding electrons are included in this "actual" structure. Resonance 1 Copyright, Arizona State University Example 3: Benzene • Each carbon is sp2 hybridized, each has 1 p A.O. • draw the pi-bonds in benzene H H H C H C C C C C H C H draw the p-bonds H H H C H C C H C H H C C C = C H C H C H "actual" hybrid structure C C C H H C C H H H EACH CONTRIBUTES 50% H H C H C • 50% of the class will draw the structure on the left, 50% the structure on the right, BOTH are required resonance contributors to the actual structure of benzene • resonance does not involve only charged structures • delocalization of the electrons in benzene in this way lowers their energy, increases stability, lowers reactivity • it is also important to be able to describe how the different resonance contributors are related using the conventional "curved arrow pushing" language of organic chemistry Allyl cation again H C H H H H H C C C C C H H H H C H H = C H C all carbons sp2 hybridized 1/2H H 1/2 "actual" structure resonance hybrid "actual" = 50 : 50 hybrid mixture of resonance CONTRIBUTORS • curved arrows show "the other place that we need to consider that the electrons could also have been" • one "double-headed" curved arrow refers to one PAIR of electrons • the allyl cation consists of a 50:50 hybrid mixture of the two resonance contributors shown (the energy of the electrons would be the same in each contributor, so each contributes equally) Allyl anion again deuterium "labels" these two H atoms H H C D H D C H C C C C D H D H H C D = 1/2 H C C D H "actual" resonance hybrid structure 1/2 all carbons sp2 hybridized "actual" = 50 : 50 hybrid mixture of resonance CONTRIBUTORS • the allyl cation consists of a 50:50 hybrid mixture of the two resonance contributors shown (the energy of the electrons would be the same in each contributor, so each contributes equally) Example Problem : Draw the resonance contributors for the following radical H H H C H C C H C C H H H C H H H C C C H C H radical H H C H H H C C C H H C H = H H C C H C H C C H H H "actual" resonance hybrid structure • resonance describes delocalization of single electrons in addition to electron pairs • note the use of a "partial electron" symbol in the actual structure and "single-headed" fishhook arrows for single electrons Resonance 2 Copyright, Arizona State University 2 Major and Minor Resonance Contributors Example : The Enolate anion H C H H O C A H H C C H H < 50% : > 50% H O >1/2 O C H C B H C H C C <1/2 H H H "actual" resonance hybrid structure = H • oxygen more electronegative, electrons would be lower in energy in contributor B compared to A • B is the major resonance contributor to the actual hybrid structure • "bigger" resonance arrowhead towards favored contributor, reflecting unequal distribution of charge between (in this case) carbon and oxygen (more than half negative charge on oxygen) • "actual" hybrid structure has all electrons that can be involved in bonding in partial (dashed) bonds. Example Problem: Draw other resonance contributors, indicate the major and minor and draw "actual" structure NH2 NH2 H3C CH2 C H3C CH2 C NH2 NH2 NH2 NH2 H3C CH2 C NH2 equal major (more bonds) minor H3C CH2 C NH2 "actual" resonance hybrid structure • the most important factor in determining the major contributor is usually whether the electrons are in a bond, the more electrons in bonds, the lower the energy • resonance delocalization stabilizes charged ions, the more resonance contributors the more stable 3 Minor Resonance Contributors to Stable Structures Many stable neutral organic molecules have resonance contributors that although are very minor, and extremely useful in predicting reactions Example: Acetone H H C O C H C H H unreasonable H O C H H C H H C H H H H major >> 50% C O C O C H H H H minor << 50% = H H C C C H H H H "actual" resonance hybrid structure Q. the second resonance contributor is clearly VERY minor, the obvious question is why even consider it? A. "because this minor contributor tells us how the electrons are unevenly distributed in the molecule, even if they are only a little uneven, as in this case • the minor structure tells us that there is a small partial charge on oxygen, and a small partial charge on the carbon of the C-O bond • note, this model gives the SAME information as the inductive effect/dipole moment model, the electrons in the C=O bond are polarized towards the oxygen O H + C C C H H H H H • "delta" symbol often used to indicate "some" charge, especially when the actual charge is very small (as in this case), or it is not obvious how much charge there is Resonance 3 Copyright, Arizona State University we will find that consideration of minor resonance contributors such as these help us to understand reactivity of many organic molecules!! Example: Phenol OH OH OH OH OH = phenol minor resonance contributors • tells us that there is a small partial negative charge on three (and specifically which three) of the carbon atoms in the ring, and that there is a small positive charge on the oxygen atom • we will use minor resonance contributors such as these a LOT throughout this course Resonance 4 Copyright, Arizona State University