Potassium
advertisement

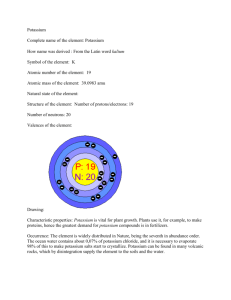
Potassium Introduction Potassium, symbol K, is an extremely soft metallic element with an atomic number of 19 and an atomic weight of 39.0983. Discovered in 1807 by the English chemist Humphry Davy, who obtained it from molten potassium hydroxide (KOH), potassium, a soft, silver-white metal, was the first metal to be isolated by electrolysis. It belongs to the family of elements known as the alkali metals. It oxidizes rapidly in air and also reacts violently with water yielding potassium hydroxide and hydrogen gas (which ignites). Because of this, potassium is stored submerged in mineral oil. It is never found alone and is difficult to isolate from its compounds. 1. What is the comic abundance of your element? Why? ppb (parts per billion; 1 billion = 109), both in terms of weight and in terms of numbers of atoms Abundance ppb by weight ppb by atoms Universe 3000 100 Sun 4000 100 Meteorites (carbonaceous) 710000 370000 Crustal Rocks 15000000 7800000 Sea Water 416000 65800 Stream 2300 59 Human 2000000 320000 About 0.1% by weight of meteorites are potassium. The average sample of the earth's crust is about 2.85 % potassium. Potassium is found in the Lithophile (crust) and not in the Siderophile (core), and therefore, will not have a great abundance in meteorites. Potassium-40 was one of the first radioactive isotopes that aided in the differentiation of the Earth. It is long-lived and in fact, it has a half-life longer than the age of the Earth, but shorter than that of our galaxy (i.e. it has yet to be broken down in the crust). 2. How many natural occurring isotopes does your element have? What are they? If there are no stable isotopes, why not? What are the longest lived- radioactive isotopes and their halflives? Potassium exists as three natural isotopes, with atomic mass numbers 39, 40, and 41. Potassium-40 is radioactive. The age of a substance can often be determined by analyzing the amount of potassium-40 it contains. The most abundant isotope is potassium-39. Several radioactive isotopes have been artificially prepared. Potassium is one of the most reactive and electropositive of metals and it is the least dense known metal. It oxidizes very rapidly in air and must be stored under argon or under a suitable mineral oil. Potassium decomposes in water with the evolution of hydrogen. It usually catches fire during the reaction with water and imparts a lilac colour to flames (see Diagram). Naturally occurring isotopes Isotope Atomic mass (ma/u) 39K 38.9637074 (12) 40K 39.9639992 (12) 41K 40.9618254 (12) Radioisotope data Isotope Mass 37K 36.9733769 38K 37.969080 40K 39.9639987 42K 41.9624031 43K 42.96072 44K 43.96156 45K 44.96070 46K 45.96168 47K 46.96551 48K 47.96551 49K 48.96745 Natural abundance (atom %) 93.2581 (44) 0.0117 (1) 6.7302 (44) Half-Life 7.63 m 11.8 x 109 y 12.36 h 22.3 h 22.1 m 17.8 m 1.8 m 17.5 s 6.8 s 1.26 s 3. What are the bonding tendencies of your element (i.e. Based on electronegativity and position in the periodic table)? Potassium has an electronegativity of 0.82, and therefore, forms ionic bonds with some elements (Cl- and Br-, for example), as well as, larger compounds (K2SO4, for example). Potassium is an alkali metal (column 1 on the Periodic Table), and has a half-filled s orbital. It is looking to lose (donate) its electron in order to have an electron configuration similar to that of the Noble Gases. 4. What is the ionic radius of your element? Is it a major element? If not, what major element will it replace during substitution? If your element is not in the standard tables, what is your estimate of its ionic or atomic radius and why? The ionic radius of K+ is 1.33 A°, which is a large radius. Potassium substitutes for other large radii. For example, in Bowen's Reaction series, potassium may substitute for iron or magnesium as you progress down the series. Potassium has a charge of +1 and can substitute for other elements with large radii according to Goldschmidt's rules for substitution. 5. Briefly, what are the chief uses by humans for your element? The superoxide KO2 is used in breathing apparatus where moisture in the breath and carbon dioxide reacts with it to release oxygen [2KO2 + H2O + 2CO2 2KHCO3 + O2 ] The alloy of potassium with sodium (NaK) is used as a heat-transfer medium in nuclear reactors. The alloy is liquid at ambient temperature and is a good reducing agent in the chemistry laboratory Potassium is used in photoelectric cells Potassium chloride, KCl, is used in preparing other potassium compounds and in fertilizers Potassium nitrate, KNO3, occur naturally in saltpeter or niter, is used in producing matches, fireworks and explosives, and has wide use as a fertilizer; also has wide use as a food preservative Potassium chlorate, KClO3, as a source of oxygen, is used in fireworks, matches, and explosives Potassium bromide, KBr, is a sedative; also used in photography, engraving, and lithography Potassium iodide, KI, promotes the discharge of mucus from the nose and from the throat, used to treat goiter and certain fungal infections; also, is added to table salt and animal feed to protect against iodine deficiency Potassium permanganate, KMnO4, is an important oxidizing agent Potassium hydroxide is used in the preparation of potassium phosphates for liquid detergents Potassium carbonate, also called potash, is used to make certain kinds of glass and soaps Potassium sulfate, K2SO4, include use as a laxative and in the production of fertilizer, rubber, and potassium carbonate Potassium cyanide, KCN, is a poison used in some insecticides and is a source for the fumigant hydrogen cyanide. It is also used to extract gold and silver from their ores Potassium chromate and potassium dichromate are used in textile dyeing and in leather tanning Electrolysis of potassium chloride yields potassium hydroxide, also called caustic potash, a water-absorbing substance used in making soaps and detergents. Caustic potash is also used for preparing many potassium salts, such as potassium carbonate, K 2CO3, a water-absorbing substance used in making glass and textile dyes and for cleaning and electroplating metals. 6. In what form does it occur naturally on or near the surface of the earth? Briefly, how is your element extracted from the earth for use in Question #5? If it does not occur naturally, how is it produced? Potassium is never found free in nature, but is obtained by electrolysis of the chloride or hydroxide. Potassium is found in nature in large quantities in various minerals such as carnallite, feldspar, and sylvite. It is part of all plant and animal tissue and essential to fertile soil. Compounds of alkali metals rank among the most common and most useful of all chemicals. Millions of tons of alkali metal salts are used by industry each year. The salts come from mines and wells. Potassium is the seventh most abundant element in the Earth's crust, making up nearly 2½ percent of the earth's crust. Large deposits of its principal compounds, including potassium chloride and potassium sulfate, occur in parts of Canada and Germany. The Dead Sea is another major source of potassium compounds. Potassium occurs in many silicate rocks and minerals. The major commercial source is salt deposits, but a small fraction is obtained from plant and animal sources. Water-soluble potassium compounds are economically recovered. They are frequently found as dry mineral deposits. Most potassium is present in insoluble minerals, making it difficult to obtain, but it can be prepared commercially by electrolysis from some refinable minerals. References: http://www.optonline.com/comptons/ceo/03843_A.html http://encarta.msn.com/index/conciseindex/1C/01CB4000.htm http://www.webelements.com World Book Millenium 2000, CD-ROM