Potassium element
advertisement
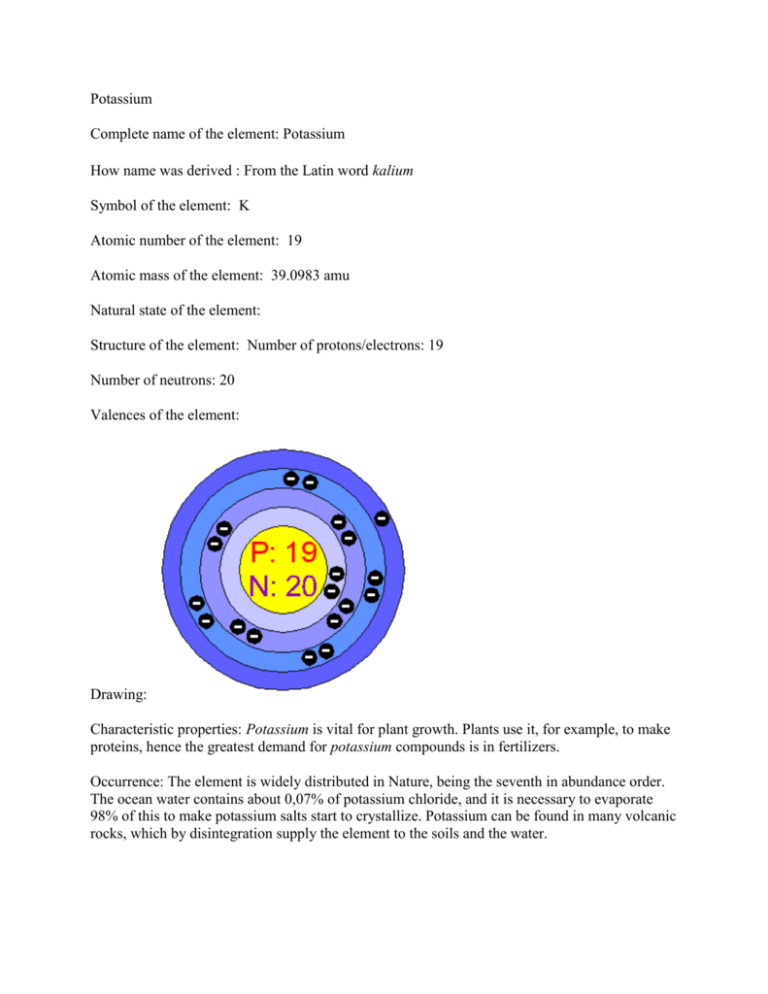
Potassium Complete name of the element: Potassium How name was derived : From the Latin word kalium Symbol of the element: K Atomic number of the element: 19 Atomic mass of the element: 39.0983 amu Natural state of the element: Structure of the element: Number of protons/electrons: 19 Number of neutrons: 20 Valences of the element: Drawing: Characteristic properties: Potassium is vital for plant growth. Plants use it, for example, to make proteins, hence the greatest demand for potassium compounds is in fertilizers. Occurrence: The element is widely distributed in Nature, being the seventh in abundance order. The ocean water contains about 0,07% of potassium chloride, and it is necessary to evaporate 98% of this to make potassium salts start to crystallize. Potassium can be found in many volcanic rocks, which by disintegration supply the element to the soils and the water. Uses: Potash is in high demand as a fertilizer. Potassium, found in most soils, is an element that is essential for plant growth. An alloy of potassium and sodium is used as a heat transfer medium. Potassium salts have many commercial uses. "Potassium." Chemicool Periodic Table. Chemicool.com. 17 Oct. 2012. Web. 2/4/2013 <http://www.chemicool.com/elements/potassium.html>. http://nautilus.fis.uc.pt/st2.5/scenes-e/elem/e01920.html www.lookchem.com/Periodic-Table/Potassium/