CH33 - Suffolk County Community College
advertisement

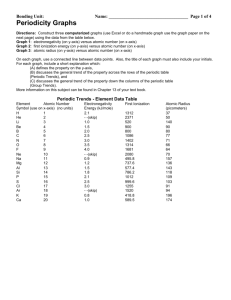
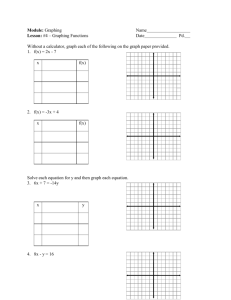
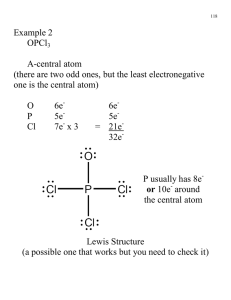
Suffolk County Community College Eastern Campus Riverhead, NY CH 33 – College Chemistry I Section 0490 Instructor: Text – Chemistry, 9th Edition, Chang (bring your text and a calculator to every class) Grading: Exams Final exam Laboratory Literature paper Attendance There will be 4 exams given during the semester, each to cover three chapters. The lowest of the 4 grades will be dropped. The final exam will be partially cumulative and is on Monday, December 18th. Bring only scrap paper, a calculator, and pencils to the exams. Once an exam has started, you may not leave the room until you have finished the exam. Cell phones may not be used as a calculator in an exam. All cell phones are to be turned off during exams. OBJECTIVES OF THE COURSE: The course is the first of a two semester course and will include the basic concepts of chemistry: atomic theory, matter, solids, liquids, and gases, bonding, trends in the periodic table, thermodynamics (energy and matter), chemical reactions, acids and bases, and solutions. WEEKLY OUTLINE Date Chapter and Subject Intro / Chapter 1 Scientific method, matter, states of matter, properties and classification of matter, standard units of measurement, scientific notation, significant figures, conversions of English to metric measurements Chapter 2 The Atom: atomic theory, structure of the atom, the periodic table, molecules and ions, chemical formulas, naming inorganic and organic compounds Chapter 3 Atomic mass, Avogadro’s number, molar mass, percent composition, empirical formulas, chemical reactions and equations, reactants and products of a chemical reaction, limiting reagents, and reaction yield. Exam Chapters 1 – 3 Chapter 4 Aqueous solutions, precipitation reactions, acidbase reactions, reduction-oxidation reactions, concentration measurements, gravimetric analysis, titrations Chapter 5 Gases, gas laws, pressure, ideal gas equation, gas stoichiometry, Dalton’s Law of Partial Pressures, deviation from ideal behavior. Chapter 6 Thermochemistry, types of energy, energy changes in chemical equations, enthalpy, heat of solution and dilution, Hess’ Law Exam Chapters 4 – 6 Date Chapter and Subject Chapter 7 Quantum theory, photoelectric effect, dual nature of the electron, quantum mechanics and numbers, atomic orbitals and electron configuration Chapter 8 Periodic relationships, the elements classified, periodic trends, variation in physical properties Chapter 9 Chemical Bonding I. Lewis dot Symbols, octet rule, ionic and covalent bonds, lattice energy, electronegativity, formal charge, resonance, exceptions to octet rule, bond enthalpy Exam Chapters 7 – 9 Chapter 10 Chemical Bonding II. Molecular geometry, dipole moment, valence bond theory, hybridization of atomic orbitals, molecular orbital theory, delocalized molecular orbitals Chapter 11 Intermolecular forces, properties of liquids, crystal structures, X-Ray diffraction, types of crystals, phase changes and phase diagrams Chapter 12 Solutions: properties, concentrations, solubility, colligative properties of electrolyte solutions and nonelectrolyte solutions Final Exam LAB OUTLINE – CH33 DATE EXPERIMENT # Check-in/Safety Requirements and Video #495 – Classifying Matter by Properties #399 – Detecting Signs of Chemical Changes #386 – Determining the Empirical Formula of Copper Chloride #477 – Balancing Chemical Equations #406 – Writing Chemical Equations and Identifying Unknown Solutions #455 – Separating and Determining the Mass of Calcium Ion in a Calcium-enriched Tablet #345 – Visible Spectrum of Hydrogen #384 – Charles’ Law #368 – Heat of Neutralization #409 – Molecular Geometry and Bonding #474 – Density, Miscibility, and Solubility Check-out Lab Reports: All pre-laboratory reports are to be completed neatly and submitted before lab begins. No late pre-labs will be accepted. All post-laboratory reports are to be completed neatly and submitted by the following lab period. Lab reports will be accepted up to one week late with an appropriate reduction in grade. Labs reports will be graded on a 1-10 scale; 3 points for the pre-lab and 7 points for the post-lab report. The lowest of your lab grades will be dropped.