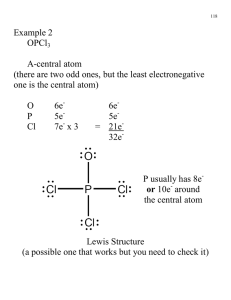
7_CHEM 163-A Problem Sets
advertisement

CHEM 163-A/General Chemistry I Assigned Problems Chapter 1 Matter and Measurement What is Chemistry? The Classification of Matter Properties of Matter Units of Measure Uncertainty in Measurement Dimensional Analysis Chapter 1 problems, pp. 30-35 (questions in red ink have answers in appendix A): 1-8, 10, 12, 14, 16, 18, 22, 28, 34(b), 36, 38, 40, 42, 46(d), 59, 70, Chapter 2 Atoms, Molecules, and Ions Atomic Theory Discovery of Atomic Structure Modern View of Atomic Structure Atomic Weights The Periodic Table Molecules and Molecular Formulas Ions and Ionic Compounds Nomenclature-Inorganic Simple Organic Compounds Chapter 2 problems, pp. 70-77: 1-6, 8, 10, 12, 14, 18, 20, 24, 28, 30, 36, 40, 42, 46, 48, 52, 54, 56, 58, 60, 66, 70, 72, 73, 75, 91, 93, 96 Exam #1 (Chapters 1, 2) Chapter 3 Stoichiometry Equations Types of Reactions Molar Mass; % Composition Avogadro’s Number; The Mole Empirical Formulas Stoichiometry Calculations Limiting Reagents Theoretical Yields Chapter 3 problems, pp. 110-119: 1-9, 12, 20, 26, 28, 30, 32, 40, 44, 50, 56, 58, 64, 68, 70, 74, 82, 84, 88 Chapter 4 Aqueous Reactions and Solution Stoichiometry Electrolytes Precipitates (displacement reactions) Acid-Base Reactions Redox Reactions Solution Concentration Solution Stoichiometry (titrations) Chapter 4 problems, pp. 157-165: 1-10, 14, 20, 22, 24, 26, 30, 34, 40, 46, 47, 48, 50, 52, 56, 60, 62, 68, 78, 84, 106 Exam #2 (Chapters 3 and 4) Chapter 6 Electronic Structure of Atoms Light Waves Quantization of Energy; Photons Line Spectra/Bohr Matter Waves Quantum Mechanics and Orbitals s, p, d, and f orbitals Many-Electron Atoms Electron Configurations The Periodic Table Chapter 6 problems, pp. 251-259: 1-8, 10, 12, 14, 16, 18, 20, 22, 26, 30, 32, 34, 36, 44, 48, 50, 52, 54, 60, 62, 64, 66, 72, 74, 89, 99 Chapter 7 Periodic Properties Historical Perspective Effective Nuclear Charge Sizes of Atoms and Ions Ionization Energy Electron Affinities Metals, Nonmetals, and Metalloids Group Trends-Metals Group Trends-Nonmetals Chapter 7 problems, pp. 291-299: 1-6, 8, 10, 12, 14, 16, 22, 24, 27, 28, 34, 36, 40, 44, 52, 54, 56, 58, 60, 64, 65, 80, 82, 91, 97, 102, 104 Exam #3 (Chapter 6 and 7) Chapter 8 Chemical Bonding Chemical Bond; Lewis Symbols; Octet Rule Ionic Bonding Covalent Bonding Bond Polarity and Electronegativity Drawing Lewis Structures; Formal Charge Resonance Structures Exceptions to the Octet Rule Strengths of Covalent Bonds Chapter 8 problems, pp. 336-343: 1-6, 8, 10, 12, 14, 16, 18, 20, 24, 26, 30, 32, 34, 36, 38, 40, 42, 46, 48, 50, 52, 54, 56, 58, 60, 62, 64, 66, 78, 85, 99 Chapter 9 Molecular Geometry and Bonding Theories Molecular Shapes VSEPR Model Molecular Polarity Valence Bond Theory Hybrid Orbitals Multiple Bonds Molecular Orbitals Chapter 9 problems, pp. 388-397: 1-10, 12, 14, 16, 18, 20, 22, 24, 26, 32, 34, 36, 38, 40, 42, 44, 46, 48, 50, 52, 54, 56, 58, 60, 62, 64, 66, 68, 70, 72 Exam #4 (Chapter 8 and 9) Chapter 10 Gases Properties of Gases Pressure: The barometer The Gas Laws The Ideal gas Equation Partial pressures Kinetic Theory; The Meaning of Temperature Effusion and Diffusion Real gases; van der Waals equation Chapter 10 problems, pp. 432-441: 1-8, 10, 12, 14, 16, 20, 22, 24, 26, 28, 30, 34, 36, 44, 46, 48, 52, 57, 58, 64, 66, 70, 74, 76, 82, 84, 95 If we have time, we will continue with Chapters 11 and 5: Chapter 11 Intermolecular Forces, Liquids, and Solids The States of Matter Intermolecular Forces Properties of Liquids Phase Changes Vapor Pressure and Boiling Phase Diagrams Structures of Solids Bonding in Solids Chapter 11 problems, pp. 476-485: 1-8, 10, 12, 14, 16, 18, 20, 24, 26, 28, 30, 32, 34, 36, 38, 40, 42, 44, 46, 48, 50, 52, 54, 56, 58, 60, 62, 64, 66, 68, 72, 74, 76, 78 Exam #5 (Chapters 10 and 11) 1 hour Chapter 5 Thermochemistry The Nature of Energy The 1st Law of Thermodynamics Enthalpy Enthalpies of Reaction Calorimetry Hess’s Law Enthalpies of Formation Foods and Fuels Chapter 5 problems, pp. 204-215: 1-8, 10, 12, 14, 16, 18, 20, 22, 24, 26, 28, 30, 32, 34, 36, 38, 40, 42, 44, 46, 48, 50, 52, 54, 56, 58, 60, 62, 64, 66, 68, 72, 80, 82 This project is funded by a grant awarded under the President’s Community Based Job Training Grant as implemented by the U.S. Department of Labor’s Employment and Training Administration (CB-15-162-06-60). NCC is an equal opportunity employer and does not discriminate on the following basis: against any individual in the United States, on the basis of race, color, religion, sex, national origin, age disability, political affiliation or belief; and against any beneficiary of programs financially assisted under Title I of the Workforce Investment Act of 1998 (WIA), on the basis of the beneficiary’s citizenship/status as a lawfully admitted immigrant authorized to work in the United States, or his or her participation in any WIA Title I-financially assisted program or activity. This product was funded by a grant awarded under the President’s High Growth Job Training Initiative, as implemented by the U.S. Department of Labor’s Employment & Training Administration. The information contained in this product was created by a grantee organization and does not necessarily reflect the official position of the U.S. Department of Labor. All references to non-governmental companies or organizations, their services, products, or resources are offered for informational purposes and should not be construed as an endorsement by the Department of Labor. This product is copyrighted by the institution that created it and is intended for individual organizational, non-commercial use only.