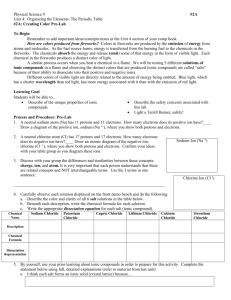
arrstrongelec
advertisement
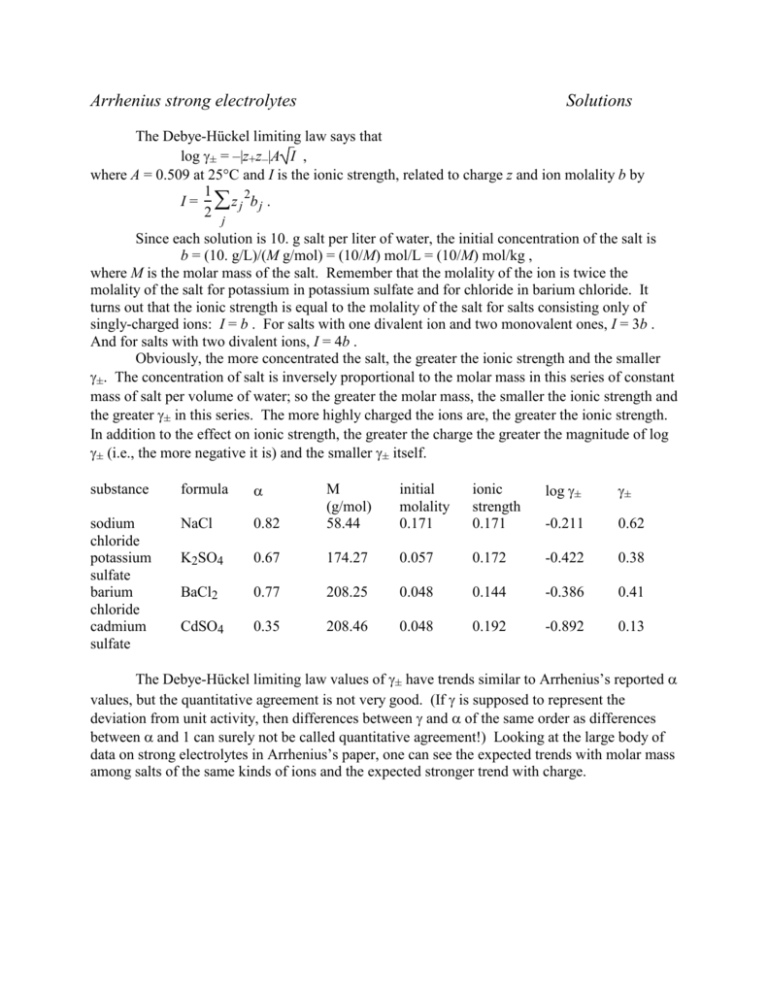
Arrhenius strong electrolytes Solutions The Debye-Hückel limiting law says that log ± = –|z+z–|A I , where A = 0.509 at 25°C and I is the ionic strength, related to charge z and ion molality b by 1 2 I = z j bj . 2 j Since each solution is 10. g salt per liter of water, the initial concentration of the salt is b = (10. g/L)/(M g/mol) = (10/M) mol/L = (10/M) mol/kg , where M is the molar mass of the salt. Remember that the molality of the ion is twice the molality of the salt for potassium in potassium sulfate and for chloride in barium chloride. It turns out that the ionic strength is equal to the molality of the salt for salts consisting only of singly-charged ions: I = b . For salts with one divalent ion and two monovalent ones, I = 3b . And for salts with two divalent ions, I = 4b . Obviously, the more concentrated the salt, the greater the ionic strength and the smaller ±. The concentration of salt is inversely proportional to the molar mass in this series of constant mass of salt per volume of water; so the greater the molar mass, the smaller the ionic strength and the greater ± in this series. The more highly charged the ions are, the greater the ionic strength. In addition to the effect on ionic strength, the greater the charge the greater the magnitude of log ± (i.e., the more negative it is) and the smaller ± itself. substance formula initial molality 0.171 ionic strength 0.171 log ± ± 0.82 M (g/mol) 58.44 sodium chloride potassium sulfate barium chloride cadmium sulfate NaCl -0.211 0.62 K2SO4 0.67 174.27 0.057 0.172 -0.422 0.38 BaCl2 0.77 208.25 0.048 0.144 -0.386 0.41 CdSO4 0.35 208.46 0.048 0.192 -0.892 0.13 The Debye-Hückel limiting law values of ± have trends similar to Arrhenius’s reported values, but the quantitative agreement is not very good. (If is supposed to represent the deviation from unit activity, then differences between and of the same order as differences between and 1 can surely not be called quantitative agreement!) Looking at the large body of data on strong electrolytes in Arrhenius’s paper, one can see the expected trends with molar mass among salts of the same kinds of ions and the expected stronger trend with charge.