NEUROLOGICAL SUPPORT - T-com
advertisement

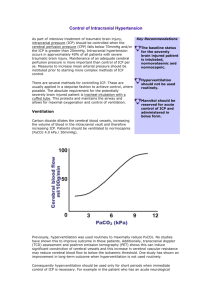
Association of North Western Intensive Care Units The General ICU Management of Patients with Severe Traumatic Brain Injury Adapted for ANWICU by Dr Charlie Granger, Lancaster Hospital and Dr Duncan Hancox, Royal Preston Hospital from the Toronto guidelines and proposed AMICU Guidelines Last Revised Oct. 2000 ICU NEUROLOGICAL SUPPORT SUMMARY This is a guideline NOT a protocol. Staff should only undertake treatments and procedures that they are familiar with or trained to perform. SUMMARY: Discuss the patient with the neurosurgeons. 1. Insert arterial and CVP monitoring lines and ICP bold / jugular bulb catheter if trained Indications for ICP placement include: a) initial GCS <9, 2. 3. 4. 5. 6. b) b) changes present on head CT, c) c) clinical deterioration Avoid hypotension at any time (Systolic Blood Pressure below 95 mmHg). Maintain CPP >70 mmHg or MAP>90mmHg if no ICP bolt Avoid hypoxia at any time (Pa02 below 60 mmHg) - PEEP is permitted Treat pyrexia prophylactically - Paracetamol 4hrly and /or active cooling to reduce hyperthermia Avoid hypercarbia (PaCO2 >40mmHg / 5.3KPa) Avoid excessive hypocarbia (PaCO2 <35 mmHg / 4.6kPa) 7. Treat abnormalities in glucose, electrolytes or coagulation 8. Ensure patient is adequately sedated with adequate analgesia 9. Paralyse patient if necessary, but only if necessary 10. Nurse patient at 15-30 degrees head-up tilt with head in neutral position 11. Avoid constriction of neck, to permit adequate jugular venous drainage 12. Avoid hypovolaemia. Use isotonic fluids or colloids. 13. Treat or avoid rises in the ICP above 20 mmHg 14. Start NG feeding early 15. Treat fitting rapidly 16. see guidelines for more details NEUROLOGICAL SUPPORT Secondary brain damage can occur at any time after the initial cerebral injury and is, to a large extent, avoidable. The aim of providing neurological support is to minimise the risk of secondary damage, by avoiding the factors that cause it, and to provide the optimum environment for recovery from the primary brain injury. Causes of secondary injury include: Systemic: Hypoxaemia Arterial hypotension Anaemia Pyrexia Hypercarbia Excessive hypocarbia Hyponatraemia Hypo/hyperglycaemia Severe arterial hypertension Intracranial: Haematoma Cerebral oedema Seizures Vasospasm Infection Hydrocephalous If secondary brain damage is to be avoided, meticulous attention to the following points is necessary: General Points Secure the airway – by definition the severely head injured will have a GCS < 9 Insert monitoring to enable patient management – arterial line, central line and ICP monitoring bolt (trained personnel only) Hypotension. In adults, hypotension is not a normal consequence of an isolated head injury, so other causes should be actively sought. A systolic blood pressure below 95mmHg doubles the risk of death after head injury. The duration of hypotension, and its severity, strongly influence the severity of secondary brain injury (1). Because of the crucial influence of arterial pressure on secondary head injury, an arterial line should be inserted in all patients with severe brain injury (GCS < 9), and a CVP line can also be very useful, especially in patients with additional injuries - i.e. major trauma. The traditional emphasis is on the Cerebral Perfusion Pressure (CPP), which is defined as the Mean Arterial Pressure less the Intracranial Pressure (ICP), but in reality the most important consideration is the cerebral blood flow (CBF). In the absence of any measure of CBF, in a head-injured patient, in whom cerebral autoregulation may be imperfect, the CPP should be maintained above 70mmHg (2). In the absence of ICP monitoring then mean arterial pressure should be >90mmHg. After euvolaemic fluid resuscitation, adequate arterial pressure should be maintained with inotropes. Noradrenaline may be the preferred choice. Hypoxia. A PaO2 below 60 mmHg is the second most powerful predictor of a poor outcome after head injury (3). PEEP of 5 to 15 cm H20 will not usually raise the ICP, but may help to reduce the risk of hypoxia (4). In a head injured patient, a GCS below 9 mandates intubation - which must be performed with the possibility of an unstable injury to the cervical spine permanently in mind (4). Pyrexia. An elevation in temperature is virtually universal after head injury, even in the absence of infection. Any pyrexia will increase the oxygen consumption of the brain, and therefore worsen the effect of hypoxia or local ischaemia, as well as increase the ICP (5). Every effort should be made to anticipate and avoid pyrexia, including regular prophylactic medication with paracetamol NG or PR. Active cooling may be appropriate if simpler measures fail to reduce the temperature below 37.5oC. Cooling below 37C probably doesn’t improve outcome other than helping to avoid hyperthermia more effectively. Hypercarbia. Carbon dioxide is a powerful cerebral vasodilator. Hypercarbia will therefore raise the ICP and worsen the effect of the primary head injury. An elevated PaCO2 should be avoided unless there are powerful reasons to the contrary. Hypocarbia. Reducing the CO2 can reduce the ICP by producing cerebral vasoconstriction. However, if the vasoconstriction is excessive this can cause a significant reduction in cerebral perfusion, further worsening the cerebral damage. Cerebral blood flow is usually reduced by up to 25% after head injury. There is now good evidence that prophylactic hyperventilation is not indicated in the first five days after head injury (6), and there is a growing consensus that during the management of head injuries the PaCO2 should not be allowed to fall below 35mmHg unless neurological deterioration while awaiting emergency CT scanning or neurosurgery, when other forms of treatment have been exhausted, and in treatment of last resort during acute deterioration (7). Electrolyte abnormalities. These may interfere with cellular function and should be avoided. Hypoglycaemia may be particularly harmful, through its effect on cellular metabolism. Blood glucose should be tightly controlled within the range 6-10mmol/l using as much insulin as required. The usual cautions on excessively rapid manipulations of the serum Na+ should be observed and Na+ should be kept within the normal range. a) Diabetes Insipidus is usually an indicator of a very grave prognosis, often only occurring after evidence of coning has developed. The consequence of non-treatment is the risk of hypovolaemia and cerebral sinus thrombosis, both of which are disastrous with a 'tight brain'. However the consequence of too early/excessive treatment is water overload leading to worse oedema and consequent organ dysfunction. Diagnostic criteria: Excessive urine output> 300ml/hr for >2hours Rising serum sodium >150 and Osmolality> 300 Low urine SG <1005 and Osmolality <300 Maintain circulating volume with nonsodium containing fluids e.g. 5% dextrose (control sugar) Treatment: Administer 1 ug IV DDAVP repeating as necessary every few hours. Smaller doses more frequently or an infusion may avoid excessive swings in urine output. b) Inappropriate ADH syndrome causes hyponatraemia due to excess water retention. – Low serum Na+ and osmolality. Positive fluid balance. Normal 24hr urinary sodium. Raised urine osmolality. Treat by fluid restriction 1.5-2 l/24 hours. c) Cerebral Salt Wasting is due to excess natriuresis. Low serum Na+. Low-normal serum osmolality Treat by sodium replacement. Coughing, restlessness and fighting the ventilator. These all raise the ICP and should be avoided by the use of adequate levels of sedation and, if required, paralysis (8). Patients should not be routinely paralyed. Some coughing on suction should be tolerated as long as any associated rise in ICP is not prolonged – this helps to reduce chest infections. Sedation should be titrated to a Ramsey score of 5-6 i.e. unresponsive to suction and pain. Neuromuscular blockade should be monitored if used, and should ideally be stopped at least once every 24 hours to observe for sedation level, fitting and to see if it is needed. Posture. The patient should be nursed at a 15 - 30 degree head-up tilt without anything, such as excessively tight tube ties, to impede jugular venous drainage (5,9). The head should be in the neutral position. Restoration of euvolaemia should precede a significant head-up posture, since the damaging effect of orthostatic hypotension on the CPP may exceed the beneficial effect of head elevation. DVT Prophylaxis A major dilemma, as patients are at high risk, but safety of pharmacological prophylaxis remains controversial. It is important to use some form of DVT prophylaxis. Suctioning and Physiotherapy These should be used to clear secretions and avoid atelectasis. They have potential to raise ICP so the patient should be well sedated. Fluid management. There is still some controversy about this. Not only is there is no strong evidence that fluid restriction has a significant beneficial effect on brain oedema, but also marked fluid restriction can result in episodes of hypotension which, as above, carry a high risk of a poor outcome. It is probably better to have produced mild hypervolaemia than to have permitted mild hypovolaemia (5). Solutions such as 5% Dextrose can increase brain water, and therefore ICP, and are probably therefore a poor choice. Beyond this, colloids or isotonic crystalloids (Normal Saline or Hartmanns solution) are equally useful. In the otherwise healthy head-injured patient, neither the urine output (particularly if Mannitol has been used) nor the CVP (especially in the presence of lung injury or mechanical ventilation) provide a reliable assessment of volume requirements. Instead, these estimations should be used in addition to physical examination and cardiovascular stability, to arrive at an overall picture (10). In patients in whom mannitol is being used to reduce the ICP it is particularly important to avoid a net fluid deficit. Hourly 1:1 replacement of urine volume and electrolytes may be required (5). Diuretics have no routine place in the management of head injury, except in the use of mannitol as an osmotic diuretic. Intracranial pressure. Ideally, all patients requiring admission to ICU because of blunt head injury and with a GCS below 9 should have an ICP monitor of some sort in place, and active treatment to reduce the ICP should be made once the ICP exceeds 20mmHg. There are a number of different monitors in use, each with its own spectrum of complications and problems. The ventricular catheters have the advantage that, in addition to measuring the ICP they allow removal of CSF to treat a raised ICP. In many cases this may be the optimal treatment for a raised ICP. In the sedated and ventilated patient, the decision to place an invasive monitor should be based on a combination of the initial clinical findings and the CT scan results or further clinical developments. Any deterioration is accompanied by a reconsideration of ICP monitor placement and re-imaging (11). Changes in the ICP can then be used as a guide for therapeutic interventions such as alterations in the blood pressure, C02 or the use of mannitol. Even better, inserting a jugular bulb catheter (a fine bore single-lumen catheter in the jugular vein that is directed up, rather than down, with X-Ray confirmation that its tip is within the skull), allows global cerebral blood flow to be assessed through changes in the jugular venous saturation, and thereby permits even finer therapeutic adjustments to the factors that control ICP and cerebral perfusion. In the absence of these monitoring devices, management of the patient should be directed at avoiding known secondary insults and maintaining a careful watch for signs of an increasing ICP - bradycardia, hypertension, widening of the pulse pressure, deteriorating GCS, focal signs or pupil dilation. Of these, an increase in pupil size is a late sign, which should not be awaited before repeat CT scans are performed. In the presence of any of these signs, Mannitol 0.5g/kg is an appropriate initial rescue therapy. Mannitol acts principally by creating an osmotic diuresis, and will not be effective if the serum osmolality is >310 mOsm. Cerebral Function Monitoring This may be the only way of detecting seizure activity or arousal from inadequate sedation in the paralysed patient. Its use is also important for the proper control of 'deep barbiturate coma' for control of intractable ICP problems. However it does require experience and knowledge to interpret the information, and therefore its appropriate use may not be practical in the DGH ITU, because of the lack of availability of expert interpretation. Feeding and Nutrition Head-injured patients require feeding. Even if their injuries appear to be relatively limited, they are in a hypercatabolic state - it is not good management to add starvation to trauma (12). Paralysed patients should be fed at 100% basal metabolic requirements and unparalysed patients at 140%. All patients should receive ulcer prophylaxis until feeding is established. After intubation an orogastric tube should be passed to decompress the stomach – this can then be used for feeding. If enteral feeding is difficult to establish then consider cisapride / metoclopramide / erythromycin or jejunal feeding tube. Other management. i. Antibiotics: The use of antibiotics will depend on any co-existent morbidity. There is no indication for prophylactic antibiotics, even in a patient with an indwelling ICP monitor. The choice of antibiotics will depend, as usual, on the presumed site of infection and the result of bacterial cultures. ii. Fitting: Patients already on anticonvulsants should be kept on them. Prophylactic use is not indicated and they should only be given if seizures occur. Fitting increases the ICP and cerebral oxygen consumption, and is therefore a potential cause of secondary head injury. It should be controlled as soon as possible, using the normal means - Diazepam IV 0.2mg/kg, followed by a loading dose of phenytoin (15mg/kg at a rate not exceeding 50mg/minute (in adults)) or phenobarbitone (l5mg/kg at a rate not exceeding l00mg/min), and then maintenance doses as appropriate (Phenytoin: l00mg 6/8 hrly, Phenobarb 50-200mg 6 hrly). For intractable seizures consider giving thiopentone boluses or infusion. iii. Calcium-channel blockers: With the exception of the use of Nimodipine in patients with sud-arachnoid haemorrhage, the potential therapeutic use of calcium channel blockers in head injured patients remains unclear (13). iv. Propofol and Barbiturates: Approx 10% of patient with severe raised ICP will not respond to the above therapies. Propofol and high dose barbiturate therapy has been shown to reduce the ICP in these patients, with a consequent small, but real, reduction in mortality. There is no indication for the prophylactic use of high-dose barbiturates. Barbiturate therapy is titrated against achieving burst suppression on the EEG, the main complications being hypotension, hypothermia, infection and prolongation of ICU admission. Boluses of thiopentone can be useful preceding or during stimulating procedures – e.g. doses of 125250mg may reduced the rises in ICP in response to physio / suctioning. Vasopressor therapy is almost always required in patients receiving high dose barbiturates (15). vi. Hypothermia: Moderate hypothermia may help to reduce the ICP -temperatures of 32 - 35 degrees centigrade. Below about 32 degrees centigrade coagulation pathways can become unreliable. If testing coagulation in a hypothermic patient, ensure that the lab performs the test at the patient's actual temperature. Transfers. Transferring head-injured patients within or between hospitals is a particularly high-risk period for the development of factors leading to secondary head injury. Every effort should be made to anticipate these factors prior to transfer, and the necessary equipment kept to hand to deal with them rapidly should they occur. Action for suspected secondary injury or raised intracranial pressure Check for: a) Jugular venous drainage is optimized b) Airway is clear c) Ventilation – minute volume, compliance, CO2 d) Oxygenation e) MAP target is being met f) Adequate sedation /paralysis if necessary (remember pain and full bladder) g) Evidence of seizures In the mean while, hyperventilate temporarily, if necessary give oxygen 100% and: a) Administer mannitol 0.5-1g/kg over 15 minutes – check circulating volume is OK b) Organize urgent CT c) Contact neurosurgeons for further advice d) Check for and correct other factors such as pyrexia, hyponatraemia etc Indications for re-scanning a) b) c) d) After 24 hours particularly if 1st scan very early (2-6hrs post injury) Sudden or persistent rise in ICP Abnormal pupil responses, lateralizing signs Prior to stopping sedation if not previously rescanned to check that signs of brain swelling have settled. Following the above points can lead to an increase in high-quality survival without a corresponding increase in vegetative patients who would otherwise have died. Suggested Intervention for the Management of Cerebral Oxygenation and Raised Intracranial Pressure Cerebral Oxygen -------------------Extraction Ratio(O2ER) = Cerebral Oxygen Consumption (CMRO2) ------------------------------------------------Cerebral Oxygen Delivery (CDO2) O2ER = SaO2 – Jugular Bulb Sv02 ------------------------------ X 100 SaO2 Cerebral O2 Extraction Ratio <40% - Maintain current PaCO2 - Observe Cerebral O2 Extraction Ratio >40% - ICP <20mm Hg ICP >20mm Hg - Decrease stimulation Sedation / analgesia / paralysis Treat any fever Hyperventilate to an O2ER no greater than 40% If ventricular drain in place, drain CSF with bag levelled at 26cm H2O Mannitol 20% 0.25-0.5g/kg bolus If repeated boluses keep serum osmolality <310mosm Repeat CT scan to exclude expanding mass lesion Increase MAP; if CVP low add volume, if CVP is OK then use Dopamine or Noradrenaline Consider barbiturate therapy (normal range 25-35% and not >40%) - Maintain Hb >10g/dl If Hyperventilated, allow PaCO2 rise until O2ER <40% or ICP >20mmHg Consider nursing patient supine Decrease stimulation Sedation / analgesia / paralysis Treat any fever Increase MAP; if CVP low add volume, if CVP is OK then use Dopamine or Noradrenaline Consider barbiturate therapy Maintain current PaCO2 Maintain Hb >10g/dl Decrease stimulation Sedation / analgesia / paralysis Treat any fever If ventricular drain in place, drain CSF with bag levelled at 26cm H 2O Mannitol 20% 0.25-0.5g/kg bolus If repeated boluses keep serum osmolality <310mosm Increase MAP; if CVP low add volume, if CVP is OK then use Dopamine or Noradrenaline Repeat CT scan to exclude expanding mass lesion Consider barbiturate therapy REFERENCES 1. Chesnut RM, Marshall LF, Klauber MR et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma 1993; 34:216-222 2. Lang EW, Chesnut PM. Intracranial pressure and cerebral perfusion pressure in severe head injury. New Horizons 1995; 3:400-409 3. Chesnut RM. Secondary brain insults after head injury: Clinical perspectives. New Horizons 1995;3:366-375 4. Abrams KJ. Ainvay management and mechanical ventilation. New Horizons 1995; 3:479-487 5. Chesnut RM. Medical management of severe head injury: Present and future. New Horizons 1995;3:581-593 6. Muizelaar JP, Marmarou A, Ward et al. Adverse effects of prolonged hyperventilation in patients with severe head injury: A randomized clinical trial. J Neurosurg 1991; 75:731-739 7 Marion DW, Firlik A, McLaughlin MR. Hyperventilation therapy for severe traumatic brain injury. New Horizons 1995; 3:439-447 8. Drielipp RC, Coursin DB. Sedative and neuromuscular blocking drug use in critically ill patients with head injuries. New Horizons 1995; 3:456-468 9. Ropper AH, O'Rourke D, Kennedy SK. Head position, intracranial pressure, and compliance. Neurology 1982; 32:1288-1291 10.Zornow MII, Prough DS. Fluid management in patients with traumatic brain injury. New Horizons1995; 3:488-498 11. Narayan R, Kishore P, Becker D et al. Intracranial pressure: To monitor or not to monitor? A review of our experience with head injury. J Neurosurg 1982; 56:650-659 12. Roberts PR. Nutrition in the head-injured patient. New Horizons 1995; 3:506-517 13. McIntosh TK, Smith DH, Garde E. Therapeutic approaches for the prevention of secondary head injury. EurJofAnaes 1996; 13:291-309 14. Smith DH, Okiyama K, Gennarelli TA et al. Magnesium and ketamine attenuate cognitive dysfunction following experimental brain injury. Neurosci Lett 1993; 157:211-214 15. Wilberger JE, Cantella D. High-dose barbiturates for intracranial pressure control. New Horizons 1995;3 :469-473