Proteomics
advertisement

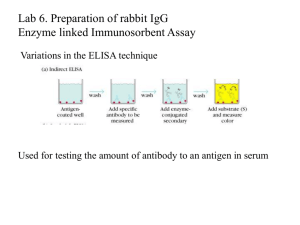
Proteomics Use Cases for FuGO (hypothetical) 1. Comparison of schizophrenic and ‘normal’ brains In this study, post-mortem samples are taken from a number of human subjects, for analysis by 2D gel electrophoresis using DIGE technology. Spots shown to differ significantly between control and test groups are then further analysed to obtain identifications. Workflow Experimental design drawn up o Pooled standard of all samples to be used for normalisation o DIGE exploited for running samples ‘2-up’ against the pooled standard, as opposed to the more normal test vs. control setup; aimed solely at running less gels (Table 1) Samples obtained for the ‘test’ group for the study on the basis of a clinical diagnosis o 10 grey, 10 white matter Samples obtained for the control group; age/sex/etc. matched against the test group o 10 grey, 10 white matter Sample prep performed, protein content assayed in all samples Control and test samples labelled with Cy3/5; Cy2 used on pooled standard 2D electrophoresis performed (a two-stage process) Gels imaged at three wavelengths (info about laser wavelength, emission wavelength and band pass emission filters can all be assumed apart perhaps from the width of the filters) Images combined and analysed by class (using particular software applications) to assess biological and experimental variation (normalised to the pooled standard) Differentially-expressed proteins identified ‘Preparative’ gel (stuffed with protein) prepared for spot picking Protein identification by LC/MS/MS o N.B. Details of parts of the machine are important – ‘equipment parts’..? Data contextualised and archived 2. Plasma and serum proteome surveys, with four-dimensional separation This collaborative project aims to identify as many proteins as possible in a stock human plasma sample (especially low-abundance proteins), by first removing the six most abundant proteins, then fractionating by pI, running fractions out on a 1D gel then doing MS on serial 2 millimetre slices of each 1D gel. Workflow Obtain sample (human plasma, or serum) o Samples were provided by the HUPO HPPP, taken (phlebotomy), stored and distributed under rigorously controlled conditions (storage tubes, courier) Removal of six most abundant proteins (albumin for example) with a MARS column Fractionation (7 for serum, 5 for plasma) in a Zoom IEF Fractionator (@ warp factor 8) Running out of each fraction on a single-lane 1D PAGE Slicing into 1mm cross sections by machine; combination into 2mm bits (mostly) In-gel digest with trypsin LC/MS/MS analysis for identification (LC = reverse phase, C18) Generation of DTA files (parameters…) and analysis with SEQUEST (more parameters…) against IPI v2.21 Plotting of gross number of IDs in a 2D grid (heat map); pI (Zoom) x M w (1D gel) 3. Localisation of organelle proteins by (ICAT) isotope tagging The assignment of proteins to particular organelles is tricky; it is essentially impossible to get a pure fraction of a particular organelle, and so this rather clever approach sidesteps the problem completely by profiling the distribution of components from particular organelles across a centrifuge gradient, then fitting the distribution of unknowns to those of known markers for particular organelles. Gels do not feature here because this is a membrane prep; most of the proteins of interest (i.e. not cargo) are membrane-bound in organelles (these do not run well on gels as they are largely hydrophobic species). Workflow Obtain sample (Arabidopsis thaliana) Run homogenised tissue on a (self-forming) iodixanol gradient Take 12 fractions (serial pipetting) Run western blots for all 12 fractions with antibody against golgi and ER markers to assess distribution across the range (sanity checking) Perform ICAT (Isotope-Coded Affinity Tagging) LC/MS/MS, with fractions separated by 3 (e.g. #3 vs. #6, #4 vs. #7, etc.) tagged one light, on heavy, to get ratios between positions in the gradient for each of the two markers, thereby deriving a distribution Compare the distribution in the same gradient of other proteins Statistically cluster with knowns to assign correct compartment o PCA, then PLS-DA (improved) 4. Other workflows One particular workflow not included here is the combination (warping) of many gel images into one master image (may be a new synthetic image, or one of the existing ones), followed by spot discovery on the master; the outlines of these spots being then unwarped to fit the originals, to allow accurate volumes to be calculated.