Chapter 6 – INTRO AND *HONORS

Chapter 6 – INTRO AND *HONORS
6.1 Chemical Bond=
Why does it occur?
Types of Bonds (An Overview)
1.
Ionic Bond
Ion=
Metal=
Nonmetal=
Want to gain or lose valence electron(s) to become like a Noble Gas
WRITING ELECTRON CONFIGURATIONS OF IONS:
1
Ionic Bond=
->usually metal + nonmetal
2. Covalent Bond
->usually nonmetal + nonmetal nonpolar covalent = polar covalent=
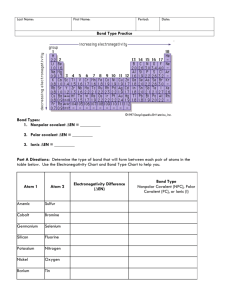
How can you tell which it is? BY ELECTRONEGATIVITY!!
Electronegativity =
Here’s an electronegativity chart:
2
If the electronegativity difference is
>1.7 =
<.3 =
.3 – 1.7 =
3.
Metallic Bond
-> metal + metal
More Details on the Bond Types
6.2 – Covalent Bonds and Molecular Compounds (#2 from Section 6.1)
Covalent Bond=
Molecule=
Diatomic Molecule=
3
Molecular Compound=
Molecular Formula=
Formation of Covalent Bonds:
*Overlapping of Orbitals (remember: want a noble gas configuration):
The Octet Rule=
4
Drawing Lewis Structures:
Single Bond=
Double Bond=
Triple Bond=
6.3
Ionic Bonding and Ionic Compounds (#1 from Section 6.1)
Ionic Bond=
Crystal Lattice=
5
Formula Unit=
Dot Structure for Ionic Bonds (remember: they want to be like noble gases):
A COMPARISON OF MOLECULAR AND IONIC COMPOUNDS:
Molecular Ionic
6.4
Metallic Bonding (#3 from Section 6.1)
Metals have LOW electronegativity
Easily give up electrons (losers)
No one is available for “grabbing”
The steps involved:
1.
donates valence electrons to the surrounding area
2.
electrons are free to move about – “electron sea”
3.
all electrons are shared by all of the metal atoms
Picture:
6
Properties of metals:
1.
2.
3.
4.
5.
TEST!!!!!!!!
6.5
Properties of Molecular Compounds (Have covalent bonds!)
A.
VSEPR =
Valence electron pairs want to be as far apart as possible!
1.
Draw Lewis Structure
2.
Look at Central Atom
3.
Count # of electron areas (bond + lone pairs)
4.
Use info from VSEPR chart below: (need to memorize for 2, 3, 4 total areas)
7
8
Examples:
Hybridization=
Explains the shapes we see – many elements do this
Carbon hybridizes to form four EQUAL orbitals
9
C is actually
C
*1s 2 2s 2 2p 2 changes to 1s 2 2s 1 2p 3
B. Types of Molecules
hybridize to form four sp
1.
Dipole = Molecule with overall charge
3 hybrid orbitals
2.
NonPolar With Polar Sites = Molecules with area of charge which cancel out
3.
Nonpolar = Molecule with no areas of charge
How Do You Tell the Difference?
Ask yourself these questions?
1.
Is there charge on the molecule?
Yes
=Dipole
Yes
2. Can it be sliced?
(so that + separated from -)
No
=NPWPS
->Non-Honors classes can use models to help them.
Examples:
No = Nonpolar
10
C. Intermolecular Forces (external bonds)
The attraction between molecules
Types of External Bonds
1.
Dipole-Dipole Bonds
Occur between :
A.
two dipoles (strongest)
Hydrogen Bond=
2.
London Force (the weakest external bond)
Occur between two nonpolar molecules (WEAKEST) or Nonpolar with
Polar Sites
The Steps:
A.
B.
C.
D.
11
Properties Based on Number/ Strength of External Bonding:
1.
State of Matter s>l>g
2.
Evaporation (*volatility) slow>fast
3.
Thickness (*viscosity) thick>thin
4.
Wetness (*adhesion)
To feel wet the substance must bond to your skin (to the Na+Cl-)
5.
Dissolving
LIKE DISSOLVES LIKE
Demonstrations:
12