Objectives 9
advertisement

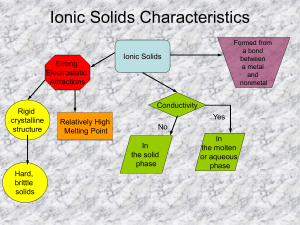
Chemistry: Writing and Balancing Chemical Equations Chapter 9 Unit Duration: Chemical reaction (rxn) Reactant Product Oxidation number Charge ions Coefficient Subscript Conservation of Matter Boot elements Diatomic Aqueous Liquid Ppt Direct combination Synthesis Decomposition Single replacement] Double replacement Combustiom Precipitate Polyatomic Cation\Anion Naming Molecular Substance and Ionic Substance Valence electrons Oxidation numbers Ions Single, double, triple bonds Octet EN δ Weeks _____________________ Objectives: The student will be able to 9.1 The Nature of Chemical Reactions 1. Describe the characteristics of a chemical reaction. (279) 2. Describe why cooking an egg portrays a chemical reaction and why substances react with each other. 3. Distinguish between reactants and products, the yield sign and delta in chemical reactions. 4. Describe how oxidation numbers (charges) determine how an atom bonds to another atom. 9.2 Chemical Equations 5. Be able to write balanced chemical reactions using words, symbols and adding the labels (l), (g), (s), (aq), (ppt). 6. Explain how a balanced chemical equation illustrates the law of Conservation of Matter. 7. Be able to write “boot elements” in chemical reactions in diatomic form when they are by themselves. 8. Describe what a coefficient and subscripts (O2 and 2O) are for when writing balanced chemical equations. 9. Be able to identify proof of reactions. 9.3 Classifying Chemical Reactions 10. Name, identify and be able to write examples of the five general types of chemical reactions both with chemicals and with the following example: Single-Replacement A + BX AX + B (yellow card for all rxn types). 11. Know how to determine if a precipitate is generated from a reaction. 12. Be able to identify, name and create polyatomic cations and anions 13. Be able to determine type of bond from Electronegativity and code for the bond type. 14. 15. 16. 17. 18. 19. 20. 21. Naming Objectives (review) Be able to draw the Lewis dot structures for any atom or any compound or ion and change to line structures. Name and Define: Molecular Substances and Ionic Substances Explain the difference between single, double and triple bonds using dots and lines. Compare and contrast polar, nonpolar, and covalent bonds. Polar substances and Non-Polar substances. Describe the octet rule for Lewis dot structures and its anomalies for Groups 1 and 2. Be able to determine valence electrons and oxidation numbers. Be able to use the Electronegativity to determine if a bond is ionic, covalent or polar covalent and code it Tell what the Delta symbol ( δ ) in a polar bond to indicates. 1