Water
advertisement

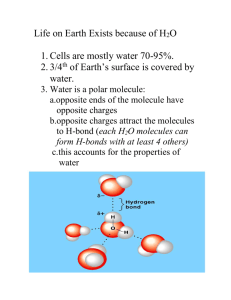
Water Life on earth probably evolved in water. Living cells are 70-95% H2O. Water covers 3/4 of the earth. Water naturally exists in all three physical states of matter--solid, liquid, and gas. The polarity of water molecules results in hydrogen bonding. Polar bonds and asymmetrical shape give water molecules opposite chares on opposite sides. Hydrogen bonding imparts a higher level of structural organization to water. Each water molecule can form a maximum of 4 hydrogen bonds with neighboring water molecules. Fig 3.1 Polarity and hydrogen-bonding impart special properties to water. Cohesive Resists changes in temperature High heat of vaporization and cools surfaces as it evaporates Expands when it freezes Versatile solvent Cohesion = phenomenon of a substance being held together by hydrogen bonds--gives water more structure than other liquids. Surface tension = measure of how difficult it is to stretch or break the surface of a liquid. Air/water interface Causes water to bead Heat and Temperature Kinetic energy = the energy of motion Heat = total kinetic energy due to molecular motion in a body of matter. Temperature = measure of heat intensity due to the average kinetic energy of molecules in a body of matter. Calorie (cal)= amount of heat it takes to raise the temperature of one gram of water one degree Celsius, also the amount of heat that one gram of water releases when it cools by one degree Celsius. Kilocalorie (Kcal or Cal) =1000 cal Water has a high specific heat, that is it resists temperature changes when it absorbs or releases heat. Specific heat = amount of heat that must be absorbed or lost for one gram of a substance to change its temperature by one degree Celsius. Specific heat of water = 1 cal per gram per degree Celsius (1 cal/g/oC Evaporative cooling Vaporization (evaporation) = transformation from liquid to gas. Heat of vaporization = quantity of heat a liquid must absorb for 1 gram to be converted to the gaseous state. Water has a high heat of vaporization at the boiling point due to hydrogen bonding. (540 cal/g or 2260 J/g; Joule = 0.239 cal) Evaporative cooling = cooling of a liquid’s surface when a liquid evaporates. Water density Water is most dense at 4oC Water contracts as it cools to 4oC As water cools from 4oC to freezing 0oC, it expands and become less dense due to hydrogen bonding--therefore ice floats Fig 3.5 Water as a solvent Solution = a liquid that is a completely homogenous mixture of 2 or more substances Solvent= dissolving agent of a solution Solute = substance dissolved in a solution Aqueous solution = solution in which water is the solvent Water is a versatile solvent because of the polarity of the water molecule. Ionic and most polar compounds dissolve in water. Non-polar compounds are not water soluble Ionic and polar substances are hydrophilic, but nonpolar compounds are hydrophobic. Hydrophilic = water loving (some large hydrophilic molecules can absorb water without dissolving). Hydrophobic = water fearing, not water soluble. Fig 3.7 Fig 3.8 There are 2 important quantitative properties of aqueous solutions: solute concentration and pH. Molecular weight = sum of the weight of all atoms in a molecule (expressed in daltons) Mole = amount of a substance that has mass in grams numerically equivalent to its molecular weight in daltons. All substances have the same number of molecules in a mole = 6.02 X 1023 (Avogadro’s number). Molarity = number of moles of a solute per liter of solution. Dissociation of water Occasionally, the hydrogen atom that is shared in a hydrogen bond with 2 water molecules can be transferred. Only a hydrogen ion (proton +1 charge) is transferred, the electron stays behind. Creates a hydronium ion (H3O+) and a hydroxide ion (OH-). H2O + H2O H3O+ + OHFig 3.UN1 By convention, ionization of H2O is expressed as the dissociation into H+ and OH-. H2O H+ + OHThis reaction is reversible, and at equilibrium most H2O is not ionized. Fig on page 47 Acid and Bases At equilibrium in pure water at 25 oC The number of H+ = the number of OH[H+] =[OH-] = 1/10,000,000 or M= 10-7 M Therefore very few molecules are dissociated (only 1 of 554,000,000). Acid = substance that increases the relative concentration of H+ of a solution. Base = substance that reduces the relative concentration of H+ of a solution. A solution in which: [H+] = [OH-] is neutral [H+] > [OH-] is acidic [H+] < [OH-] is basic Strong acids and bases dissociate completely in water e.g. HCl and NaOH. i.e. HCl H+ + ClWeak acids and bases dissociate only partially and reversibly e.g. NH3 (ammonia) and H2CO3 (carbonic acid) pH Scale In any aqueous solution [H+]X[OH-] = 1.0 X 10-14 In a neutral solution both = 10-7 M In a basic solution where [H+] = 10-9 M, the [OH-] = 10-5 In a acidic solution where [H+] = 10-5 M, the [OH-] = 10-9 pH scale = scale used to measure degree of acidity (ranges from 0 to 14) pH = negative log10 of the [H+] expressed in moles per liter. pH of 7 is neutral pH < 7 is an acid solution pH > 7 is a basic solution Most biological fluids are within the pH range of 6 to 8 (some exceptions e.g. stomach acid with a pH of 1.5) Each pH unit represents a tenfold difference (scale is logarithmic), so a small change in pH represents a large change in actual [H+]. Fig 3.9 Buffers Buffer = substance that minimizes large sudden changes in pH---helps organisms maintain the pH of body fluids within a narrow range. Buffers are combinations of H+ donor and H+ acceptor forms in a solution of weak acids or bases. They work by accepting H+ ions from a solution when they are in excess and by donating H+ ions to the solution when they have been depleted. e.g. Bicarbonate buffer H2CO3 H+ donor (weak acid) HCO3+ H+ acceptor (weak base) H+ Hydrogen ion