200715141828Thakaresonalicsuschemconfabstract
advertisement

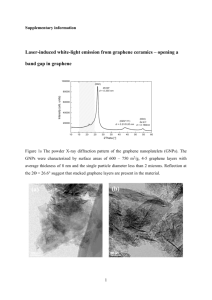
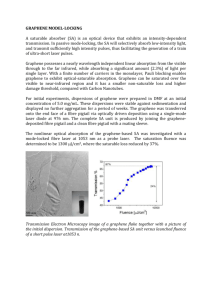
SusChemE 2015 International Conference on Sustainable Chemistry & Engineering October 8-9, 2015, Hotel Lalit, Mumbai __________________________________________________________________________________ Amino functionalized graphenes as base catalysts in condensation reactions S.C.Thakare, R. V. Jayaram* Department of Chemistry, Institute of Chemical Technology, Matunga, Mumbai-400019 Presenting author email address:thakaresonalic@gmail.com Corresponding author email address: rv.jayaram@ictmumbai.edu.in __________________________________________________________________________________________________ 1. Introduction: Graphene, a form of carbon consisting of planar sheets which are one atom thick, has generated enormous excitement due to its unique properties such as large thermal conductivity, superior mechanical and electrical properties [1]. The use of metal free catalysts based on carbonaceous materials is an area of current interest. Heterogeneous carbon materials used as catalysts in chemical synthesis are termed as carbo-catalysts. Graphene based materials such as graphene oxide (GO) are considered as a new class of carbo-catalysts and have opened a series of novel applications in chemical synthesis. One possible route to enhance the application of graphene in catalysis is to modulate its properties by chemical doping and modification. For example, amino functionalized graphene was found to have enhanced catalytic properties than intrinsic graphene. In this work, amino functionalized graphene is prepared from GO by Solvothermal method [2] and characterized by elemental analysis, FTIR, XRD, SEM and TGA studies. The surface basicity was determined by temperature programmed desorption (TPD) using CO2 as probe molecule. The functionalized material exhibits good activity and reusability as solid base catalysts in Knoevenagel condensation reaction. 2. Materials and Methods: 2.1 Materials and reagents Graphite flake (99%, 325 mesh), H2SO4 (98%), NaNO3 (98%), H2O2 (30wt %), ammonia solution (25%), HCl (36 %), KMnO4 were procured from S. D. Fine Chemicals, India and used without further purification. 2.2 Preparation of graphene oxide Graphene oxide was synthesized using Hummers Method [3]. In this method, concentrated H2SO4 was added to a mixture of graphite flakes (3.0 g) and NaNO3 (1.5 g), and the mixture was cooled to 0 °C. KMnO4 (9.0 g) was added slowly by keeping reaction temperature below 20 °C. After complete addition of KMnO4 the reaction mixture was heated to 35 °C with vigorous stirring for 30 min. Then appropriate amount of water was then slowly added by maintaining reaction temperature at 98 ⁰C for 30min. Additional water and 30% H2O2 were added, to produce the exotherm. The mixture was kept to cool at room temperature. The mixture was neutralized by distilled water and GO was collected by centrifugation and then dried it at 80 ⁰C. 1 2.3 Preparation of amino functionalized graphene (NH2-G) 100mg of GO was added to 40ml of ethylene glycol under ultra-sonication. After addition of 1ml of aqueous ammonia, the solution was transferred to a Teflon lined autoclave for a solvothermal reaction at 180⁰C for 10h. After completion of the reaction the precipitate was filtered, washed repeatedly with distilled water, and dried at 60⁰C for 24h. 2.4 Catalytic activity In a typical run, 24 wt% of catalyst is added in 1mmol benzaldehyde ,1.2mmol ethylcyanoacetate,4.0 ml of anhydrous toluene and stirred at 80⁰C for 4h. Progress of the reaction was monitored by TLC .The percentage conversion of benzaldehyde was determined by GC analysis. 3. Result and discussion: The introduction of nitrogen in GO was confirmed by elemental analysis. (Table 1) Sample GO G-NH2 Table 1.Elemental Analysis of GO and NH2-G C (%) H (%) 39.087 1.467 39.760 2.906 N (%) ----2.863 The presence of –NH2 groups was confirmed by IR spectroscopy Fig .1 FTIR spectra of GO and NH2-G The presence of amino group generates basic sites on graphene, determined by TPD analysis. NH2–G shows desorption peak in the range 600⁰C-800⁰C which confirms the presence of strong basic sites of amino groups (Fig.2)..Amount of basic site on amino graphene is 0.281mmol/g. 2 231587 (A) Signal ( mV ) 200 (B) 100 0 -100 0 200 400 600 800 Temperature / °C Fig.2 NH3-TPD profile of NH2-G (A) and CO2-TPD profile of NH2-G (B) TGA curve of GO and NH2-G are shown in Fig 3. GO shows three main weight loss steps. The first weight loss ~5% around 100⁰C is due to the evaporation of water. The second weight loss of 60% around 100-210⁰C is due to labile oxygen groups and residual water. The third weight loss ~ 5 to 7% occurs between ~450-550⁰C and is due to the pyrolysis of stable oxygen groups such as phenol and carbonyl. After amine functionalization the weight loss between 100-210⁰C is reduced to 10%, which suggests that most oxygen group on GO are reduced. NH2-G GO NH2-Gr Fig 3.TGA curves of GO and NH2-G 3.1 Catalytic activity of NH2-G Fig.4 Schematic representation of Knoevenagel condensation between benzaldehyde and ethylcyanoacetate This study was further extended by testing catalytic activity of amino graphene over various substituted benzaldehyde using ethylcyanoacetate and malononitrile. 3 3.2 Reusability of the catalyst % conversion of benzaldehyde Reusability of amino graphene in the condensation reaction was tested by carrying out repeated runs with the same batch of the catalyst. After each cycle, the catalyst was filtered off, washed with acetone and dried at 60⁰C. It was observed that the activity of amino graphene did not decrease even after five cycles (Fig 5). 71 70.9 70.8 70.7 70.6 70.5 70.4 70.3 70.2 70.1 70 1 2 3 4 5 Recycle Number Fig 5. Re-usability of the catalyst 4. Conclusions: 1) Amino functionalized graphene was prepared by simultaneous reduction and functionalization of GO using aqueous ammonia by solvothermal method. 2) The amino graphene shows better thermal stability than GO. 3) Amino graphene exhibited good catalytic activity in Knoevenagel condensation reaction and the material is reusable up to five cycles. 4) There is ample scope for using the functionalized grapheme in various other base catalyzed reactions. References [1] Y.Zhu,S.Murali,W.Cai,X.LI,J.W.Suk,J.R.Potts,R.S.Ruoff,,Adv.Mater.22,2010,3906-3924 [2] L.Lai,L.chen,D.Zhan,L.Sun,J.Liu,S.H.Lim,C.K.Poh,Z.shen,J.Lin,Carbon 49,2011,3250-3257 , [3] W.S.Hummers,R.E.Offeman,J.Am.Chem.Soc.80,1958,1339. 4 5