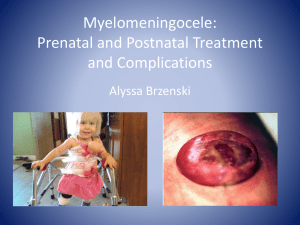
بسم الله الرحمن الرحيم - Sudan University of Science and Technology
advertisement

بسم هللا الرحمن الرحيم Sudan University of Science & Technology College of Veterinary Medicine Department of Veterinary Medicine & Animal Surgery Theriogenology II (Obstetrics & Gynaecology II) 5th class year Course Contents 1- Caesarean Operation in farm animals. 2- Dystocia (causes types and treatment). (i) Maternal dystocia. 3- Disorder associated with parturition. (i) Injuries and disease incidental to parturition. (ii) Post-parturient prolapse of the uterus. 4- Infertility in female of farm animals. The caesarean Section (Hysterotomy) In the cow: C.S is the removal of the fetus through an incision in the abdominal wall, at the time of parturition, when it can not be delivered in the normal manner. It is considered as a routine obstetric procedure in cattle practice which has high maternal and fetal survival rates and is less exhausting, faster and safer than fetotomy. The goals of the cesarean section are preservation of the dam and calf and the future reproductive efficiency of the dam. Indications: 1- Fetomaternal disproportion: i. Physical immaturity of the dam: It is not uncommon for heifers to be parturient at term at only 14 months of age. Even at 18 months of age, the maternal pelvic is still immature and usually too small for vaginal delivery. ii. Fetal oversize: In many cases of oversize, the fetal head cannot draw into the maternal pelvic cavity. iii. Fetal monsters and infection. iv. Post maturity: Prolongation of pregnancy up to 290 days. 2- Incomplete cervical dilatation: Is a common cause of dystocia in cattle, but it should be diagnosed only after careful assessment of the findings on vaginal exploration (by vagina scope). Slow or arrested, dilatation in multiparous cows may be associated with uterine inertia caused by hypocalcaemia. Incomplete dilatation of the cervix is an important complication of uterine torsion. 3- Irreducible uterine torsion: Torsion of the uterus in cattle constitutes a major indication for a caesarean operation, either because the torsion is irreducible or because the cervix fails to dilate after correction. In the most cases of post-cervical torsion, the degree of cervical dilatation and vaginal twisting permits the introduction of a hand into the uterus for manipulation of the fetus, but if the torsion affects the cervical canal or uterine body, the fetus is totally inaccessible. Such torsion is an absolute indication for a C.S. The combination of uterine displacement and oedematous swelling of it wall may well result in perforation of the uterine body, especially by the fetal head. 4- Fetal monsters: Such as: schistosoma reflexus, Achondroplasia (bull calf deformity) and fetal dropsy cause dystocia. 5- Faulty fetal disposition: Provided that the cervix is fully dilated and remain so; most early cases of faulty fetal disposition can be corrected manually or relieved by relatively simple fetotomy. However, the loss of fetal fluids followed by uterine contraction often makes these manipulations difficult and time-consuming and more likely to result in rupture of the uterus. 6- Fetal emphysema: Fetal emphysema is a frequent for complication of protracted parturition in cattle and, irrespective of the primary cause of dystocia; it is often the immediate indication for a C.S. Such cases should be assessed realistically before the operation is undertaken because fetal putrefaction can seriously influence maternal survival. 1 7- Miscellaneous indications: The natural termination of pathologically prolonged pregnancy may also be associated with absence of normal parturient changes in the vagina and vulva and a consequent need for a C.S. Abortion in late pregnancy sometimes requires treatment by a C.S for several contributory reasons, such as incomplete birth canal dilatation and cervical constriction. Fetal mummification and hydrops uteri may now be treated initially by inducing parturition, but a C.S may still be necessary if induction fails. Laboratomy is essential in cases of uterine rupture. Restraint: A caesarean operation may be performed with dam standing, or in sternal, lateral or dorsal recumbency. The choice depends on the surgeon's preference, demeanour of the animal and available facilities. For standing surgery, the animal should be restrained using a halter. The halter should be tied with a quick-release knot in case of recumbency. Sedation should be avoided if possible because, it can cause recumbency during surgery, ruminal tympani and may be detrimental to fetal survival. If sedation is necessary, xylazine is commonly used (0.05-0.1 mg/kg I/M). Two or more assistants are usually required for successful surgery: as a minimum, one to restrain the cow and one to deliver the calf. The location for surgery should be selected carefully with the objectives of ensuring good hygiene, lighting, and facilities for restraint a suitable floor surface. Anaesthesia: The choice of anaesthesia methods varies between surgeons and selected surgical site. For flank incisions, paraverterbral anaesthesia of nerves associated with transverse processes of T13, L1, L2, and L3 is indicated (20 ml of 2-3% lignacaine with adrenaline). The advantage of paravertebral anaesthesia is that the whole flank musculature is desensitized and flaccid, which facilitates exploration of the abdomen during surgery and closure of the wound. Also the flank incision can be extended readily if necessary during surgery. One disadvantage is that the technique is more difficult to perform than other methods. A local anaesthetic line block or inverted-L block of the flank is indicated. An 18 gauge needle is used to administer 2% lignacaine with adrenaline at several sites. At each point, 5 ml of local anaesthetic is injected S/C in each direction of the incision line, and further 10 ml into the musculature. The technique is quick and reliable, and requires minimal training. However, the parietal peritoneum may not be effectively anaesthetized, causing reaction by the patient when it is incised. A similar reaction will occur if the incision has to be extended during surgery. Furthermore, because the flank is not flaccid, apposition and suturing of the muscle layers can be difficult, and there may be an adverse effect on wound healing. Epidural anaesthesia using lignacaine can provide adequate anaesthesia of the flank (such anaesthesia tends to cause recumbency). Pre-operative preparation: A wide surgical field should be prepared. Initially, dirt and dust should be brushed from the field and back of the animal before the operative field is clipped and shaved. The skin should be prepared using a surgical scrub (7.5% povidone iodine) following by surgical spirit. Sterile drapes should be applied; in the standing animal a large single 2 drape (laboratomy sheet) with a suitable window can be placed over the back of the cow and down the flank. Surgeons and assistants should wear protective surgical scrub suits (surgical gowns with long-sleeved plastic gloves and surgical gloves). Operative technique: Left flank incision is the most common technique and is most appropriate for the standing position. One advantage of the left flank incision is that the rumen can be used to prevent exposure of the intestines. Another advantage of the flank incision in the standing animal is easier correction of uterine torsion.Finally, wound dehiscence is more manageable in the flank, compare with lower abdominal incisions. A vertical skin incision is made in the middle of the flank starting 10cm ventral to the transverse processes and extending approximately 30-40cm long. Alternatively, a slightly oblique incision from caudo-dorsal to cranio-ventral, about 30° from vertical can be used, starting 10 cm from the tuber coxae. The advantage of oblique incision is that the internal abdominal oblique muscle can be split along its fibres and there is improved access to the genital tract. A ventrolateral incision is particularly indicated for the removal of an emphysematous fetus. The cow should be in right lateral recumbency. An oblique incision, starting from the flank fold dorsal to the attachment of the udder, is continued cranially, parallel to the ventral border of the ribs. The advantage of this approach is that it gives good exposure of the uterus, even when it is friable, and it minimizes the risk of uterine contents contaminating the abdominal cavity. However, repair of the abdominal muscle layers can be more difficult if the muscles are under tension and sutures may tear through the tissues. A surgical drain may be inserted during repair of wound. A midline or paramedian incision is not commonly used in the field because general anaesthesia or heavy sedation is required and respiratory function of the dam is compromised. However, the technique gives excellent access to the uterus. A non absorbable suture should be used for repair of all muscle layers of the incision because postoperative wound dehiscence has severe implications, including herniation. A right flank incision is uncommon; however, it is indicated if the left flank approach is obstructed by adhesions as result of previous surgery. Access to the uterus is good, but the small intestines are difficult to retain within the abdomen and they interfere with the surgery. With left flank approach the following muscle layers are incised: cutaneous, external abdominal oblique, internal abdominal oblique and transverse abdominal muscle. They are incised using a scalpel, unless the fibres can be split parallel to the skin incision. The peritoneum is incised using a scalpel, taking care not to puncture the rumen. The incision can be extended using scissors, rather than a scalpel, to reduce the risk of cutting abdominal organs. The uterus should be exteriorized by grasping and applying traction to a distal extremity of the calf (hind leg). To aid exteriorization of hind limb the calf's foot can be held using the surgeon's right hand and the hock with the left hand, so levering the foot up through the incision. Exteriorization of the uterus prior to incision is a critical step in the subsequent success of surgery. If the calf is in the right uterine horn, it will be necessary for the surgeon to rotate the uterus along it is longitudinal axis to bring the calf's limbs to the flank wound. Rotation can be achieved by traction on the leg with left hand, whilst pushing the dorsal aspect of the uterus away from the surgeon with the flat of the right hand (A similar technique can be attempted in case of irreducible uterine torsion). 3 The uterine wall incised over the calf's leg from toe to hock by scalpel or scissors. Care should be taken to avoid incising the calf, particularly if the fetal fluids are sparse. In addition, the surgeon should avoid incising cotyledons, which can lead to profuse haemorrhage. The allantochorion and amnion are ruptured manually, and the calf's fetlocks grasped by the surgeon, exteriorized and passed to an assistant (may use sterile ropes or chain). Initially, in the case of forelegs, both the legs and the head should be exteriorized by the surgeon. Then the calf is extracted by assistants whilst the uterus is held by the surgeon. Initial exteriorisation of the hind limbs is done dorsally and then caudally, once the calf's pelvis emerges (a similar way to per vaginam delivery of calf in posterior presentation). The emphysematous fetus presents unavoidable risks of peritoneal contamination. In such cases, incision of the uterus is often followed immediately by the escape of gas and fetid fluid; parts of the fetus may be grossly swollen and crepitate on handing. The uterine wall is often tightly stretched, and intrauterine manipulation can be difficult. Such fetus often requires considerable traction with sharp or blunt hooks applied in the orbits and at appropriate points on the trunk or upper limbs. It may be necessary to incise deeply at several sites over the thorax and abdomen to release gas. A live calf should be immediately attended to by an assistant, whilst the surgeon examines the uterus, initially for presence of a second fetus. In addition, any lacerations of the uterine wall should be noted and repaired. The fetal membranes are removed if they can be readily detached (uncommon). Otherwise, they are returned to the uterine lumen and any protruding tissue trimmed. This approach is justified on two grounds. Firstly, it should be assumed that if the fetal membranes can physically be separated, they will be expelled naturally and more completely by uterine contractions. Secondly, if deliberate detachment of the fetal membranes is attempted before they would normally separate and be expelled, then there may be haemorrhage or incomplete removal either of microvilli or larger masses of placental tissue. It is common practice to place antimicrobial pessaries in the uterine lumen before repair of uterine incision. The edges of the uterine incision are inspected for haemorrhage; particularly from the cotyledonary vessels (large vessels should be ligatured). The uterus is supported by assistant or held using uterine forceps (grasping forceps) and the incision sutured using catgut or ployglactin 910. Catgut has advantages over synthetic suture materials particularly when the uterus is friable. A variety of suture patterns have been employed; all are inverting patterns such as Cushing's and Lembert's. Once the uterine incision has been repaired, the surface should be cleaned with sterile gauze and/or normal saline to removed blood clots and other debris and returned to its corrected location within the abdomen, ensuring that there is no torsion of the genital tract. Oxytocin (20-40 iu) may be administered I/M to hasten uterine involution at this point. The administration of water-soluble antibiotic such as crystalline penicillin, within abdominal cavity is recommended by some surgeons. The peritoneal cavity should be closed as quickly as possible to reduce chance of bacterial contamination. The abdominal flank incision should be repaired in three layers: peritoneum and transverse abdominal muscle, internal oblique muscle and external oblique muscle (a continuous suture pattern is used). Antibiotic may be infused between each muscle layer. The skin is repaired using nylon in a horizontal mattress or simple interrupted suture. Post-operative Care: The calf should be dried and navel dressed with antiseptic immediately after calving. 4 Once surgery is completed, 2-3 litres of colostrums from the dam should be administered to the calf. The wound should be cleaned following surgery. Oxytocin should be administered I/M to stimulate further uterine involution. Calcium borogluconate should be administered I/V to prevent hypocalcaemia and facilitate uterine involution. A non-steriodal anti-inflammatory agent should be considered, at least in cases of severe dystocia, uterine torsion or uterine infection prior to surgery. If there is evidence of surgical shock, I/V fluid therapy is indicated; 2-3 litres of hypertonic sodium chloride are particularly effective. Administration of antibiotic 3-5 days. The dam is often re-examined 24-48 hours after surgery and note of the temperature, demeanour, appetite and faecal consistency should be noted. Skin sutures are removed 3 weeks after surgery. Complications of Caesarean section: 1- Subcutaneous emphysema: Air often leaks from the abdominal cavity into the s/c tissues and muscle layers following surgery, if the peritoneum is not closely apposed, causing emphysema. It has no significant detrimental effect on the animal and treatment is not required. 2- Metritis and retained placenta: Removal of the membranes during surgery is rarely possible. However, if they are retained more than 24 hours after surgery, gentle attempts at removal can be made daily by exploration of the vagina only. Intrauterine and I/M antibiotic can be administered, and once the membranes have been expelled, gentle lavage of the uterine lumen with 5 litres of warm, normal saline can be administered. 3- Peritonitis: Inadequate repair of the uterine incision, particularly in the presence of a metritis, is the main cause of postoperative peritonitis. The incidence is increased in the case of a dead or emphysematous fetus, after severe dystocia, rupture of the uterus or presence of a fetal monster, and after spillage of uterine fluids into the abdomen during surgery. Diarrhea, pyrexia, inappetence and abdominal pain are common presenting signs of peritonitis following C.S. A variety of treatments have been suggested including parenteral antibiosis, intraabdominal administration of antibiotic through the right flank and I/V fluids therapy. 4- Wound dehiscence: Predisposing factors of wound dehiscence include inadequate asepsis, low abdominal incision and trauma to the tissue during surgery. In addition, removal of skin suture too early after surgery. Serum-like fluid occasionally accumulates at the ventral aspect of the wound between the muscle layers if the dead space is not occluded and will resolve spontaneously or can be drained surgically. In other cases, there may be formation of an abscess. 5- Postpartum haemorrhage: Haemorrhage from the abdominal incision is usually limited. However, haemorrhage from the uterine incision can be considerable and in some cases fatal, if the cotyledonary vessels are disrupted. Occasionally the haemorrhage may be minimal at surgery, but may progress in the 24 hours following operation. 5 Prevention is by careful incision of the uterus, supporting the genital tract adequately during surgery, and attention to haemostasis. Treatment of severe haemorrhage is by a blood transfusion. 6- Nerve paralysis. 7- Fractures. Post-operative fertility: Post-operative productivity implies not only the maintenance of bodily condition and on an acceptable level of lactation but, also the ability to conceive again and sustain a developing fetus to term. The calving interval is increased in cows following a C.S compared with normal calving. Reduced fertility may occur as a consequence of increased incidence of retained fetal membranes and endometritis, uterine adhesions that hinder involution and reduced endometrial tissue competence. In addition, there is an increased frequency of abortions during subsequent pregnancies, possibly as a result of scar tissue formation within the uterine wall limiting expansion of the uterus and/or nutrition of the fetus. In the mare: Because it is not often necessary, the caesarean operation in the mare is still widely regarded as a serious and, by inference, dangerous. In fact, the mare tolerates this surgical interference as well as most other species and the generally good recovery rate after a caesarean operation has largely disproved the myth that the horse’s peritoneal cavity is exceptionally vulnerable to infection or the development of dangerous postoperative adhesions. Indications: a) Faulty fetal disposition that cannot be corrected by other means (e.g. transverse presentation) b) Vulvovaginal or uterine trauma. c) Vaginal oedema. d) Irreducible uterine torsion. e) Severe congenital deformities (wryneck, ankylosed limbs, hydrocephalus). Anaesthesia: Anesthetics always pose some risk to the mare and foal. Most drugs given to broodmares can have potential adverse cardiopulmonary effects on the fetus. Therefore, administration of the anesthetic agent least likely to compromise the mare and foal is recommended. The goals of general anesthesia are: provide narcosis, relieve maternal pain, have minimal anesthetic time and provide for rapid, safe maternal and neonatal recovery. Pre-anesthetic agents given to the mare should cause minimal depression of the foal. Xylazine is preferred over a promazine derivative as blood pressure is less likely to be depressed. The recommended dosage of xylazine is 0.25-0.5 mg/kg IV or 0.5-1.0 mg/kg IM. Administration of 1.5 to 2.0 mg/kg ketamine is used for anesthetic induction. A relatively insoluble inhalation and anesthetic gas such as isoflurane or sevoflurane should be used to maintain anesthesia. Advantages of gas anesthetics are that they are short-acting, they tend to increase cardiac output and they are less likely to promote uterine bleeding. Once anesthetized, the mare should be monitored as she becomes recumbent. At this time, aseptic preparation should begin immediately. 6 Operative technique: The operation can be performed through a midline, paramedian or ventral flank laparotomy. The midline approach is now widely adopted for gastrointestinal surgery and is even more satisfactory for caesarean section because this operation considerably reduces intra abdominal pressure, and the wound can therefore be repaired easily without excessive tension on the sutures. The mare’s uterus is seldom so tightly contracted that a fetal limb cannot be grasped through the uterine wall and brought through the abdominal wound. For this reason a uterine incision of adequate length is easily made on the greater curvature of the gravid horn with little risk of tearing during manipulation of the fetus. The fetus is then extracted making maximum use of joint flexibility and gently supported outside the abdomen with its umbilical cord intact. The equine fetus is less sensitive than the fetal calf to ‘pinching’ stimuli in utero and, unless the placenta is separated, fetal viability should be assumed until cord or heart palpation proves otherwise. The uterine incision is repaired with polyglycolic acid inversion sutures in one or two rows, depending on whether the first row of stitches tears through the uterine wall, which is sometimes noticeably fragile. After the removal of clotted blood and other debris, a soluble antibiotic preparation may be sprinkled on the uterine incision. The peritoneum and sub-peritoneal fat need not be stitched. The laparotomy repair is completed with a continuous subcutaneous suture and appropriate stitches in the skin. Postoperative management: Oxytocin should be administered to induce uterine contraction even when the placenta has been removed at surgery. Antibiotic therapy is generally considered advisable pre- and postoperatively, especially if the foal has been dead for some time, resulting in putrefaction. Wound infection is treated by removal of appropriate skin sutures to provide drainage. Postoperative fertility: The caesarean operation in the mare should not have too great an effect on subsequent fertility provided it is performed quickly after the onset of dystocia, and before there if heavy bacterial contamination of the uterus either from manual interference or from putrefaction of the foal. In the doe goat and ewe: Indications: I. Failure of the cervix to dilate. II. Irreducible or severely traumatized vaginal prolapse. III. Feto-pelvic disproportion, particularly in primiparous animals with a single fetus. IV. Fetal emphysema after protracted dystocia. Anaesthesia: Hysterotomy is usually performed through a left flank incision under paravertebral, inverted-L nerve block or local infiltration analgesia with the animal in right lateral recumbency, using 2–3% lignocaine hydrochloride with adrenaline. Operative technique: 7 The left sublumbar region is close-clipped and the skin prepared for aseptic surgery. The skin is incised in the mid-sublumbar fossa, and the underlying muscles are incised in the same way as described above for the cow. However, it is important to stress that the body wall is very much thinner, and great care must be taken not to incise into the rumen accidently. A fetal extremity, preferably the hock, is grasped through the uterine wall so that an incision can be made in the same way as that described for the cow; however, it is important to stress that more than a single fetus is likely to be the norm. It is more important to remember to explore the uterus, particularly the opposite horn to that incised, to ensure that all lambs have been removed before suturing the uterine incision. It is always possible to remove all lambs through a single incision. The fetal membranes should be removed if they can be readily detached; if not, then that which cannot be returned to the uterine lumen, thus interfering with the closure of the uterine incision, should be excised. The uterus should be closed using a single inversion suture pattern such as Lembert’s or Cushing’s, using an absorbable material. The sheep, more than any other species, is highly susceptible to the toxaemic effects of intrauterine clostridial infection, and most deaths are due to this complication. Postoperative fertility: Caesarean section is an effective method of resolving dystocia in sheep and goats and does not adversely affect subsequent fertility. In the bitch: Indications: In larger animals the cause of dystocia can usually be identified, but this is often not possible in the bitch. Frequently the decision to operate is therefore based largely on a subjective assessment of the circumstances of the case including: o The duration and progress of parturition. o The number and viability of fetuses born and unborn. o The nature of vulval discharges. o Changes in the pattern of straining. o The often uninformative findings on vaginal examination. Hysterotomy is done on an emergency basis for cases of dystocia refractory to medical management. Indications of C.setion in the bitch are: i. Uterine inertia not responding to medical treatment. ii. Obstruction. iii. Fetal distress (Ultrasonography).Decreased fetal heart rate (below 150/min.) Inhomogeneous amniotic fluid. iv. Dead pups, fetal malformations. v. Overdue bitch (after day 67). vi. Vaginal infection. vii. Pregnancy toxicosis. viii. Elective c-section Anaesthesia: For premedication, phenothiazine tranquillisers are very useful agents since they smooth anaesthetic induction and reduce the subsequent dose of induction and maintenance agents. 8 For the induction of anaesthesia, the ultra-short acting barbiturates and propofol (2-4 mg/kg IV) appear to be most useful, since they are either rapidly redistributed or are metabolised, and therefore have limited effect upon the fetuses after delivery. For maintenance of anaesthesia, the volatile agents are preferable, such as isoflurane. This agent has a rapid uptake and elimination by the animal and it may have a better cardiovascular margin of safety than the more soluble agents such as halothane. A local anesthetic administered as a line block or via epidural can decrease the amount of inhaled anesthetic needed to keep the patient anesthetized. Operative technique: The ventral approach allows the incision to be made as cranially as necessary, and allows equal exposure of the two uterine horns. The length of incision depends upon the expected size of the fetuses; ideally it should be sufficiently large to enable the uterus to be exteriorized. Speed of the surgery is important for two reasons: to ensure minimal fetal hypoxia and to prevent hypotension of the dam caused by compression of the caudal vena cava by the gravid uterus. Once the linea alba is incised, care should be taken not to damage the uterus which may be lying in close apposition to this structure. Once the uterus has been identified, it should ideally be exteriorized and packed off using swabs to prevent contamination of the abdomen with fetal fluid. However, care must be taken when manipulating the gravid uterus, which has a thin wall and is liable to tearing. The uterus should be incised in a relatively avascular area of the dorsal surface of the uterine body, although in some cases a ventral incision may be made. The latter is usually necessary when there is impaction of a fetus that prevents exteriorization; a ventral incision leads to peritoneal contamination with fetal fluid. Once at the incision, the amniotic sac may be ruptured and fetal fluids should ideally be removed by suction. The umbilical vessels should be clamped approximately 2 cm from the ventral abdominal wall of each pup and the umbilical cord can then be severed distally. Once fetuses have been delivered, they should be passed to an assistant for resuscitation. At this time, the pups should be inspected for congenital abnormalities such as cleft palate, and if necessary the cord can be ligated with suture material. After each pup is delivered, the associated placenta should be removed by gentle traction or by gentle squeezing of the uterine wall and twisting of the cord; those that are firmly adherent should be left in position, since forceful removal will result in haemorrhage. Attached placenta will be expelled by uterine involution, supplemented by exogenous oxytocin administration after the termination of the procedure. It is important to ensure that all fetuses are removed, and careful inspection of both uterine horns up to the ovaries and the uterine body is essential. The uterus and the broad ligament should be assessed after delivery of all pups; small traumatic lesions should be identified for subsequent repair. The uterine incision is usually closed using a two-layer inverting continuous pattern such as Cushing or Lembert with an absorbable suture material. 9 There should have been minimal peritoneal contamination, but if this has occurred the peritoneum should be lavaged with several litres of warmed physiological saline. Omentum may be placed on to the region of the uterine incision to reduce the likelihood of adhesion formation. The abdominal incision and skin should be closed in the normal manner Postoperative management: The puppies’ prime requirement immediately afterbirth is not food but warmth and the maintenance of an ambient temperature of 30–32°C. Two particular problems may require veterinary attention during the initial postoperative period. a) A continuing vulval discharge of blood may indicate serious haemorrhage from areas of placental attachment, especially if placentae have been forcibly detached. This is a life-threatening complication, especially in animals of a small size, and indicates the need for further oxytocin therapy immediately. b) The second cause for concern may be the persistence of compulsive panting or hyperventilation, to the extent that it interferes with the bitch’s natural inclination to suckle the puppies or even to sleep. It is occasionally caused by the unnecessary provision of extra heat from an overhead lamp or other appliance, but most often it develops spontaneously in bitches, especially of the brachycephalic types, which have behaved in a similar way during the first and second stages of parturition. Like all other species, the bitch is susceptible to infective peritonitis after caesarean operation, but good surgical technique and routine antibiotic therapy minimize the risk of this complication. Postoperative fertility: There are no data on postoperative fertility in the bitch, but it is certainly high, probably because the ovary and oviduct are completely protected by the bursa and are unlikely to be affected by adhesions. In the queen cat: The indications for caesarean operation in the queen are not well documented, and it is likely that gravid ovariohysterectomy is performed more frequently than hysterotomy. Inertia and oversize are less common in this species than faulty disposition, or fetal deformities such as hydrocephalus and anasarca. Maternal causes of dystocia include pelvic distortion after fractures and uterine torsion affecting either the entire uterus or only one horn. The operation is performed under general anaesthesia using similar considerations to those described in the bitch. The surgical approaches and technique described for the bitch are equally suitable for the queen. Antibiotic and supportive fluid therapy is advisable after protracted dystocia or if the uterus is grossly infected. 10 Dystocia Dystocia means difficult birth (Greek word for normal birth is eutocia). Consequences of Dystocia: The consequences of dystocia are numerous, and will depend upon the severity. Dystocia result in: Increased stillbirth rate and mortality of the offspring. Increased neonatal morbidity. Increased mortality rate for the dam. Reduced productivity of the dam. Reduced subsequent fertility and increased chance of sterility. Increased likelihood of puerperal disease in the dam. Increased likelihood of culling. Causes of dystocia: Obstetricians have usually regarded dystocia as being either maternal or fetal in origin; however, there are sometimes occasions when it can be difficult to identify the primary cause. More realistically, dystocia should be considered in relation to defects in the three components of the birth process: the expulsive forces, the adequacy of the birth canal, and the size and disposition of the fetus. Incidence of dystocia: In the cattle, the dystocia is more common in primipara than in pluripara, and more common with birth of male than female calves, and also with twins. Cows calving in winter are more likely to experience dystocia than those calving in summer, probably because of lack of exercise. Many dairy producers, in an effort to reduce the incidence of dystocia, resort to using beef bulls, especially on heifers, because the resulting calf is thought to be smaller. While this practice may reduce dystocia in the short run, it is costly in the long run. In the Sheep, the incidence of dystocia is influenced by breed, ranging from 1% in Scottish Blackface to 77% in the Texel. In the goat the frequency of dystocia is generally low. In the horses, the incidence of dystocia is low. All studies have shown that dystocia occurs more frequently in primipara than in pluripara. Obstetrical Terminology: 1- Presentation: signifies the relation between the longitudinal axis of the fetus and the maternal birth canal. It is includes longitudinal presentation, which can be anterior or posterior depending on which fetal extremity is entering the pelvis; transverse presentation, ventral or dorsal according to whether the dorsal or ventral aspect of the trunk is presented; and vertical presentation (is very rare), ventral or dorsal. 2- Position: indicates the surface of the maternal birth canal to which the fetal vertebral column is applied. 3- Posture: refers to the disposition of the movable appendages of the fetus and involves flexion or extension of the cervical or limbs joints: for examples, lateral flexion of the neck or hock flexion posture. The approach to an obstetrics case: Each case of dystocia is a clinical problem which may be solved if correct procedure is followed. History of the case: Many points will be elicited from personal observation of the animal a) Has full term arrived or is delivery premature? b) Is the animal a primigravida or multigravida? 11 c) d) e) f) g) h) i) j) k) What is her previous breeding history? What has been the general management during pregnancy? When did straining begin? What was its nature? Has straining ceased? Has a water bag appeared and, if so, when was it first seen? Has there been any escape of fluid? Have any parts of the fetus appeared at the vulva? Has an examination been made and has assistance been attempted? In the case of polytocous species, have any young been born, naturally or otherwise, and if so, when? Were they alive at birth? l) Is the animal still taking food? General Examination: The animals’ physical and general condition should be noted. If recumbent, is she merely resting or is she exhausted or suffering from a metabolic disease? Body temperature and pulse rate should be noted. Parts of a fetus may be protruding and it may be possible to asses the nature of the dystocia from these. Are exposed fetal parts moist or dry? May be nothing protrudes from the vulva, in which case particular attention should be paid to the nature of discharge. Fresh blood (profuse), generally indicates recent injury to the birth canal. A dark brown fetid discharge indicates a grossly delayed case (the fetus is dead). Detailed Examination: The animal should be effectively restrained for the safety of the veterinarian, any assistants and the animal concerned in a clean environment. In the case of the mare, cow, ewe and doe goat it’s easier if they remain standing. Very rarely it may be necessary to sedate the dam (if she is very fractious). The clean hot water with soap or surgical scrub should be available for washing and reduce contamination of the genital tract as possible. With an assistant holding the tail to one side. The operator, having washed his hands and arms and after donning a clean disposable plastic sleeve, proceeds to make a vaginal examination. If on examination the vagina is found to be empty, attention should be directed to the cervix. Is it completely effaced? If its not, is it partially dilated and is it still occupied by some sticky mucus? If so, then it may be concluded that the 1 st stage of labour has not yet begun, and the animal should be given more time. Can a fetal tail and anus be identified? If so, it is highly probable that the case is one of breech presentation. In the mare, complete emptiness of the vagina from the membranes may be due to postural defects (dorso-transverse presentation). The protrusion of the allantochorion into the vagina and from the vulva- 'red bag'- indicates placental separation. In the majority of cases some part of fetus occupies the vagina. The head, a limb or limbs. Recognition of the head is not difficult (mouth and tongue). In the case of a limb, the first requirement is to ascertain whether it is a forelimb or hind limb. If the planter aspect of the digit is downwards, it is highly probable that it is a forelimb; the converse is equally true. Proof is obtained by nothing the direction of flexion of the limb joints. If the joint immediately above the fetlock flexes in the same direction as the latter, the limb is fore one. If two limbs are present, it must be established that they are either fore or hind, and if they are from the same fetus. In the protracted case, assessment of the exact nature of the dystocia and correction may be more difficult. Often, particularly in heifers and mares the vaginal wall becomes grossly swollen and oedematous so that the insertion of a hand and arm becomes difficult. Loss of fluids has resulted in the fetal parts and mucous membrane becoming dry. Plenty of obstetrical lubricant is required. 12 Consideration of Treatment: The great majority of dystocia cases in the monotocous species are fetal in origin (faulty disposition or oversize). In the former, the first aim of treatment is to convert it to normal, and having done this, hasten delivery by relatively gentle traction. Such correction must be performed (if possible) by manipulation, assisted perhaps by the use of simple instruments such as snares and repellers. In the cases of oversize of fetus a decision must be made quickly on whether to attempt delivery by traction or by a C.S. Fetotomy as methods of treating dystocia in large animals still has its place if the fetus is dead. Maternal Dystocia: Dystocias, which arise in the mother due to maternal factors, are caused either by constriction of the birth canal or by deficiency of expulsive force. Constriction of the birth canal: 1- Pelvic constriction: An inadequate pelvis is very common cause of dystocia in bovine primipara. The pelvis is late maturing compare with some other aspects of skeletal development. Pelvic constriction followed fracture, where has been poor alignment of the pelvic bones, can be an important cause of dystocia in any species. 2- Incomplete dilatation of the cervix: It is common cause of dystocia in sheeps, goats and cattle. The degree of incompleteness of dilatation varies from virtually complete closure, to small obstruction. In cattle, incomplete dilatation may occur in both the heifer and multiparous cow. It is more likely to be due to hormonal dysfunction (PGF2α and oestrogen). Incomplete dilatation of the cervix frequently accompanies uterine torsion. Incomplete dilatation of the cervix of the ewe and doe goat is descriptively named ‘ringwomb’. Manual exploration of the birth canal reveals that the cervix is in the form of a tight, unyielding ring which will admit only one or two fingers (may be dilated by digital manipulation). Many cases of the ringwomb in ewes follow preparturient prolapse of the vagina. 3- Incomplete relaxation of the posterior vagina and vulva: This is a relatively common finding in dairy heifers. It seem to be associated with heifers which are overfat body condition, or in herds where the animals have been moved just before calving, or where the process of calving has been interrupted by too frequent observations or interventions. Treatment requires the patient application of slow and gentle traction. If excessive force is used, perineal damage will be occurring. If the vulva will not dilate properly then episiotomy should be carried out. If there is any doubt about the likelihood of success with continuing attempts at vaginal delivery, a C.S should be performed. 4- Vaginal cystocele: This name given to condition occasionally encountered in the parturient mare and cow in which the urinary bladder lies in the vagina or vulva. It is of two types: Prolapse of the bladder through the urethra. It is more likely to occur in the mare due to the great dilatability of the urethra and force of straining efforts. Protrusion of the bladder through a rupture of the vaginal floor. In this condition the bladder will lie in the vagina (the serous coat of the organ will be outermost). It is important to differentiate both conditions from protrusion of fetal membranes; particularly in the mare. Treatment: Induction of epidural anaesthesia with or without sedation. Invert the prolapsed bladder again by manipulation. 13 In the case of a protruded bladder, it must be replaced through the tear in the vaginal wall and the latter sutured. 5- Neoplasms: Neoplasm of vulva and vagina may occur in all species and causes physical obstruction. In the cow, papilomata, sarcomata and submucous fibromata of the vagina and vulva occur, while in the bitch the vaginal submucous myxofibromata is common. Neoplasms of the cervix are rare in animals as the causes of dystocia. 6- Pelvic obstruction by the distended urinary bladder: The birth canal may be obstructed by distended urinary bladder being forced back by straining in the form of mound under the vaginal floor. Careful catheterization of the bladder relieves the condition. 7- Torsion of the uterus: Torsion of the uterus, or part of it, is seen as a cause of dystocia in all species. In the cattle: Rotation of the uterus on its long axis, with twisting of the anterior vagina, is a common cause of bovine dystocia. Aetiology: 1) Instability of uterus with result from the greater curvature of the organ being dorsal, and the uterus being disposed cranially to its suspension by the ligaments. 2) The violent fetal movements which occur in response to the increasing frequency and amplitude of uterine contractions during 1st stage of labour. 3) Ecessive fetal weight is also a predisposing factor. 4) Uterine torsion may occur when the cow is attempting to rise to her feet from sternal recumbency, particularly when she is in a confined space. Clinical features: The torsion in an anticlockwise direction is more common than in the other direction (75%). Although the uterus rotates about its longitudinal axis the actual twist in the majority of cases involves the anterior vagina; in the minority of cases in which the twist affects the posterior parts of the uterus there is minimal distortion of vaginal wall. The severity of the twist does not directly affect the survival of the fetus, fetal death being caused by loss of fetal fluids or separation of the placenta. The most constant feature of uterine torsion is it’s associated with parturition. It’s generally the torsion occur during the 1st stag of labour, because immediately after correction the cervix is found to be dilated to a variable degree. However if after correction the cervix is found to be fully dilated or, if before correction, the membranes or fetal parts are protruding through the cervix, the inference should be that the torsion occurred during early 2nd stage of labour. Symptoms: Restlessness due to sub acute abdominal pain associated with myometrial contractions and cervical dilatation. In severe cases of torsion there may be increasing restlessness and more probably all parturient behaviour will cease. If the condition is unrelieved, the placenta will separate and the fetus will die. There will develop persistent low-grade abdominal pain, progressive anorexia and constipation. Secondary bacterial infection of the fetus will develop due to the fetal membranes remain intact. 14 Diagnosis: Diagnosis is made by palpating the stenosed anterior vagina, whose walls are usually disposed in oblique spirals which indicate the direction of the uterine rotation. The cervix palpable by carefully following the folds into the narrowing vagina, the lubricated fingers can usually be pressed gently forwards and through the dilated cervix. In torsion of less than 180° portions of the fetus may enter the vagina. Treatment: With the adoption of prompt treatment, prognosis is favourable for mother and fetus. Delay leads to fetal death and makes treatment more difficult. The possible forms of treatment are as follows: a) Rotation of the fetus per vaginam: The aim of this method is to reach the fetus by insinuation of the hand through the constriction of the anterior vagina and partially dilated cervix and then to apply a rotational force to the uterus through the medium of the fetus. It is likelihood of success depends mainly on two factors: whether the cervix is sufficiently dilated to admit the hand and whether the fetus is alive. When the fetus is reached, purchase is obtained on its shoulder or elbow region in order to rotate it in the opposite direction of the twist, but the first manoeuvres are designed to generate a gentle swinging motion in the fetus before attempting to reduce the torsion. It is helpful to have the rear of the cow at a higher level than the front, and epidural anaesthesia should be beneficial. b) Rotation of the cow's body (correction by 'rolling'): This was the most popular method of correction, but because it requires at least three assistants it is being replaced by the previous method. The principle of this method is to roll the cow around it is uterus while that organ remains still. The cow is cast on the side to which the torsion is directed. Thus in an anticlockwise torsion she is cast on her left side. The two fore legs and two hind legs are tied together and the head is restrained with a halter or head collar. The cow is rolled sharply over onto her other (right) side. The patency of the vagina is checked and if the torsion persists the cow is gently rolled back onto her other (left) and process is repeated. The efficiency of rolling can be improved by putting external pressure on the cow’s abdomen in an attempt to ‘hold the uterus still’ while the cow body is rolled. (Schaffer plank method). c) Surgical correction: If the case cannot be corrected by either of the previous methods, a laparotomy should be performed on the standing cow through the left or right sublumber fossa an attempt made to rotate the uterus by intra-abdominal manipulation. The caesarean operation is indicated if the torsion is irreducible or if the cervix is insufficiently dilated or fails to dilate further after reduction. Expulsive Deficiency: The expulsive force of labour comprises the combined forces of myometrial contractions and straining (abdominal contraction). 1- Primary uterine inertia (spontaneously): Primary uterine inertia implies an original deficiency in the contractile potential of the myometrium, thereby removing or reducing this component of the expulsive force and delaying or preventing the completion of the 2nd stage of labour. It is a common cause of dystocia in polytocous species (49% of dystocia in the bitch and 37% in queen cat). It occurs in the cow, where it is usually due to hypocalcaemia, hypomagnesaemia. Causes: The changes in progesterone: oestrogen ratio. The progesterone: oestrogen ratio is important as it influences uterine contractility in a number of ways. Oestrogen 15 increases the synthesis of contractile protein; number of agonist receptors for oxytocin and PGF2α. Progesterone has the opposite affect, thereby reducing myometrial contractility. Oxytocin and prostaglandins deficiencies. Calcium and magnesium deficiency will impair uterine contractions and causing inertia (due to transients decline in food intake around the time of calving). Overstretching of the myometrium due to presence of a large litter or excess fetal fluids (hydrallantois), or understretching due to a small litter in polytocous can cause reduced uterine activity. Adiposity/ fatty infiltration between the layers of the myometrium can reduce it is contraction. Hereditary and senility. Systemic illness. Diagnosis: Case history. Examination of birth canal and presenting fetus. The animal is at term, as denoted by mammary changes and ligamentous relaxation in the pelvis, while psychological (behaviour) manifestation, coupled with restlessness due to abdominal discomfort, will have indicated that the 1st stage has passed. Treatment: a) In the large monotocous species, treatment is generally simple. By vaginal manipulation the membranes are ruptured, and if the fetus is in the normal disposition, it should be delivered immediately by traction. b) In cows, Ca borogluconate should be given even if there is no clinical evidence of hypocalcaemia. c) In the bitch and queen may be treated by: i.Vigorous exercise of the dam will sometimes stimulate uterine contractions. ii.Digital stimulation of the vagina will stimulate endogenous oxytocin release, and may induce uterine contractions. iii.Slowly inject 10% calcium borogluconate solution i.v. (0.5-1.5 ml/kg body weight). iv.Leave the bitch for 30 minutes; if straining commences then repeat the calcium borogluconate treatment. If not, administer oxytocin at dose rate of 0.5-5 iu i.v. or 1-10 iu i.m. depending on size, and 0.5 iu i.v. or i.m. in the queen. v.Perform a vaginal examination and remove any pups or kittens by gentle traction. vi. C.S should be indicated if the previous treatment is not success. Secondary inertia (dependently): This is inertia of exhaustion and is essentially a result of dystocia due to some other cause, usually of an obstructive nature. In polytocous species prolonged unsuccessful efforts to deliver one fetus may result in dystocia from inertia to others fetuses. Secondary inertia is frequently followed by retention of the placenta and delayed uterine involution (predispose to puerperal metritis). Sometimes in the bitch and queen cat, normal parturition will commence but after expulsion of a few pups or kittens will then cease, even though there is no obstruction (‘primary partial inertia’,). In the monotocous species, correction of the dystocia which provoked the inertia is the essential feature of treatment. In the bitch when the case is of longer duration, and there are still several young to be born. It is best to proceed with delivery of the remainder (used Hobdays forceps). 16 Fetal Dystocia: The two main division of fetal dystocia are feto-maternal disproportion and faulty fetal disposition. Fetomaternal Disproportion: Is a common cause of dystocia in all species, particularly in the cattle (oversize of fetus). Cattle: Is the common cause of dystocia in heifers. Calf birth weight: Calf birth weight is the single most important factor affecting the incidence of dystocia. Each kilogram increase of birth weight increased of the rate of dystocia by 2.3%. A number of factors have been shown to affect calf birth weight; they are as follows: 1- Breed of sire: In cross-breeding programmes, the selection of the most appropriate sire breed is important for ease of calving and low calf mortality rate. In general it has been found that when the parents are of disparate size, e.g. Friesian bull and Jersey cow, the birth weight of cross calf is near the mean of body weight for purebred Friesian and purebred Jersey calves. When the cross is made between the beef sire and dairy heifer or cow, the incidence rate of dystocia may will be increased. 2- Parity of dam: A very simple rule is: the bigger the dam, the bigger the calf. But it also occurs with breeds with heifers giving birth to smaller calves than parous cows. 3- Sex of calf: Many studies have shown that the birth weights of male calves are greater than female calves. 4- Seasonal and climatic factors: The mean spring birth weights of calves born after a warmer were 4.5 kg lower than those following cold winter. One hypothesis for this finding is that, during cold winters, there is increased uterine blood flow which result in an increased nutrient supply to the fetus. 5- Nutrition of the dam: The influence of maternal nutrition during pregnancy on development and health after birth, as well as on birth weight, is associated with the influence of under nutrition during the early stages of gestation when the placenta is developing. 6- Length of gestation: Certain fetal calf developmental abnormalities such as adrenal-cortex hypoplasia or aplasia have been associated with prolonged gestation. The increased gestation length is associated with higher birth weight and an increased chance of dystocia. 7- In vitro maturation and fertilization: There are numerous reports that the birth weight of calves originating from this source is greater than those following normal artificial insemination. Calf conformation: Some fetal monsters, such as fetal duplication, schistosomes, and ascetic calves are prevent normal expulsion (despite the weight of calf is low). Maternal factors: 1- Body condition score of the dam: It is generally, that heifers or cows in a very high condition score are more likely to suffer from dystocia than those that are moderate to poor, the reason being that 17 those in very good condition will have a substantial amount of pelvic fat, which will reduce the size of the birth canal. 2- Pelvic capacity of the dam: Although the Friesian-Jersey calves were larger in proportion to their dams than purebred Friesian calves; the incidence of dystocia with purebred Friesian calves was about three times the incidence for the Friesian-Jersey calves. These indicate that the Jersey cow has a more favourable pelvic capacity than the Friesian. Pelvic area is moderately to highly heritable (about 50%), and thus can be used as a measurement in the genetic selection of breeding stock. Prevention of Dystocia due to Fetomaternal disproportion: 1- Management at service: Ensure body weight at time of service is more than 260 kg. In natural service, select a bull with a record of easy calvings, and avoid bulls of large breeds. In artificial insemination, select a well-proven bull of high genetic merit and it has been used successfully on heifers on several farms. 2- Management before calving: Adjust feed levels to avoid calving in an overfat condition. Restrict energy intake in the last 3 weeks of pregnancy. Check iodine and selenium levels if calf mortality has been high in previous years. Ensure supplementary magnesium is provided. Ensure that the adequate exercise area is available. Observe the heifers at least 4-5 times daily during the last 3 weeks of pregnancy. 3- Management at calving: Housed heifers should calve in familiar surroundings and avoid moving them to a calving box. Observe hourly when calving starts. Too frequent observations can delay calving. Be a good stockperson is trained to identify potential problems (abnormal pain and signs of fear) and be ready to assist if calving is prolonged. Faulty fetal Disposition: 1- Presentation: In the monotocous species, serious dystocia always occurs with posterior presentation if the hindlimbs are flexed; even when they are extended there is a greater likelihood of dystocia than with anterior presentation. Because of the relatively long limbs of the fetuses, and the large space required for hindlimb extension. In ovine twin births, breech presentation causes dystocia. In polytocous births, when the hindlimbs are flexed the incidence of dystocia is increased. In the mare, transverse presentation causes the most serious dystocia. 2- Position: As regards position of the fetus, the natural tendency is for it to lie with dorsum against the greater curvature of the uterus so as to occupy as little space as possible: thus the equine fetus is upside down and the bovine fetus is upright during late gestation. The latter maintains this relationship during birth, but in the mare the fetus changes from a ventral to a dorsal position during the birth, and this it may be cause dystocia in mare. 18 3- Posture: In the monotocous species, the dimensions of the maternal bony pelvic are just sufficient for the normal full-term fetus; any fetal disposition other than anterior presentation, dorsal position and extended posture is likely to result in dystocia. In polytocous species the feto-maternal relationship is not so exact, with the result that the disposition of the comparatively small fetal limbs is less important and many puppies and kittens are delivered normally with their limbs in postures which would have caused dystocia in the foal and calf. Manipulative delivery per vaginam: Obstetrical Equipment: Simple instruments that are easy to handle and convenient to sterilize are best. More complex equipment is occasionally required. Some of instruments are used in cattle include: 1- Obstetric snares: meter length, with loops of cotton rope, nylon cord, a finer cord for snaring the mandible is essential- and traction bars. 2- Moore's obstetric chains with handles (may be easier to use than the rope snares). The main advantage is that they are heavier and do not move so readily when they are repositioned during intrauterine or intravaginal manipulation. 3- A snare introducer. This can be used with rope as well as chain. 4- Obstetrical hooks include Krey-Schottler double-jointed hooks, Obermeyer's anal hook, Harms's sharp or blunt paired hooks on a fine chain (farrowing), and Blanchard's long flexible cane hook. These are useful when performing fetotomy and traction to be applied to various fetal segments. 5- Additional instruments are Cammerer's torsion fork with canvas cuffs and Kuhn's obstetrical crutch. Instruments for fetotomy include: Fetotomy knifes such as Robert's or Unsworth's (S/C fetotomy). Spatula for use in S/C fetotomy. Fetotome, with wire introducer, wire, hand grips. Gattli's spiral tube, which are a cheaper alternative of protecting the dam's genital tract than the fetotome. Obstetric Manoeuvres: 1- Retropulsion: Retropulsion means pushing the fetus cranially from the vagina towards the uterus. It is affected by pressure with hand on presenting bulk of the fetus. In others Retropulsion is applied by means of a crutch. As far as possible, the repelling force should be applied in intervals between bouts of straining. Epidural anaesthesia may be induced to prevent the straining. 2- Extension: Extension refers to the extension of flexed joint when postural defects are present by hand or snare or hook. 3- Traction: Traction means the application of force to the presenting parts of the fetus in order to supplement or in some cases to replace the maternal forces. Such force is applied by hand or through the snares or hooks. Limbs-snares are fixed above the fetlocks, and the head snare may be applied by the Benesch method, in which the loop is placed in mouth and up over the pool and behind the ears or center of the a single rope may pushed up over the pool and behind both ears, leaving both ends of the rope protruding from the vagina. For replacement of the laterally deviated head, where the operator's hand is insufficient, a thin rope snare applied to mandible is essential. 19 A very important consideration is the magnitude of the supplementary force. This may be used, since excessive force applied can cause severe trauma to dam and fetus. The most important aspect of applying effective traction is to coordinate the supplementary force with the straining effort of the dam. In the case of the cow, the slack in the calving snares is 'taken up' as she strains so preventing the calf from returning to its original site within the birth canal. In the mare, the use of snares with several persons is usually sufficient. In the ewe and doe goat traction can apply by using simple fine cord snares or a fixed plastic head snare. In the bitch and queen cat, the best instruments are the fingers, whelping forceps and may use vectis. 4- Rotation: Rotation is alteration of the position of a fetus by moving it around its longitudinal axis; for examples, from the ventral to dorsal position. It more often required in horses than in cattle and is much more easily effected on the responsive live fetus, which may be rotated by digital pressure on the eyeballs, protected by the lids. If this fails and in the case of dead fetuses, fetal fluid supplements are indicated- rotational force may be applied on the crossed extended limbs by hand or by Cammerer's torsion fork or Kuhn's crutch. 5- Version: Version means alteration of transverse or vertical to longitudinal presentation. Obstetric Anaesthesia for vaginal delivery: 1- General Anaesthesia: Deep narcosis or general anaesthesia is better suited to the temperament of mares than local anaesthesia. Using hobbles and a hoist, it is easy to place the mare in dorsal or lateral recumbency. Such a change of position may greatly ease obstetric manoeuvres; in addition, elevation of the hind quarters will allow the foal to fall back into the uterus in the abdomen, there by providing more space for and manipulative procedures. 2- Epidural Anaesthesia: Cattle: Is ideal for obstetric purpose. This form of anaesthesia is useful when-ever straining is troublesome, as in prolapse of the uterus, vagina, rectum or bladder. It is facilitating intra-vaginal manipulation. The site of injection is the middle of the 1st intercoccygeal space. This is located by raising the tail 'pump handle' fashion to identify the first obvious articulation behind the sacrum. The sacrococcygeal space can also be used; however it is smaller than the first coccygeal space and in some older cows becomes ossified. A dose rate of 1 ml/ 100 kg of 2% lignacaine hydrochloride (heifers and small cows 5 ml, large cows 7-10 ml) injected. Sheep and goat: Caudal epidural anaesthesia is a very useful. The injection can be made into either the sacrococcygeal or the 1st coccygeal interspace using 2% lignacaine with adrenaline at dose rate of 1 ml/ 50 kg body weight. Horses: Because the root of the tail is well covered by muscle and fat, the spines of all coccygeal vertebrate are not so easy to locate; the 1st coccygeal interspace is the preferred site. A dose rate of 6-8 ml/ 450 of lignacaine injected. 20 Treatment of dystocia due to disproportion Fetomaternal disproportion: anterior presentation: In heifers this can be due to a failure of the posterior vagina and vulva to dilate; in adult cows it is often associated with to great a bulk of fetal chest and shoulder at the entrance to the maternal pelvis. Vaginal delivery: For vaginal delivery, three snares are required; a loop is made in the head snare (Benesch method), and each of the other snares is placed above the fore fetlock of the calf. At first, traction is applied to one foot snare with a view to advancing one shoulder at a time through the pelvic entrance. Then the other leg is advanced. All three ropes are then pulled on. At all times traction should be synchronous with straining, as far as practicable, initial pulling should be upwards; once the head engages the vulva, however, the direction of traction should be obliquely downward. Frequent application of lubricant to the vagina and fetus are indicated. If the vulva is relatively small and further traction on the calf will cause rupture of the vulva and perineum, episiotomy should be performed. Fetotomy: Fetotomy (embryotomy) is sectioning of a dead fetus into two or more parts within the uterus and vagina to reduce the size and that delivery through the birth canal becomes possible .The method used involves the removal of one or sometimes two forelimbs, to reducing the fetus. If the head is likely to impede the manipulation it may be returned to the uterus; failing this, it may first be removed. A foreleg may be removed by subcutaneous or percutaneous fetotomy. Subcutaneous fetotomy: The essential instrument is fetotomy knife. The leg is snared-around the pastern and sustained traction applied to it by an assistant. The obstetrician makes a small incision with a scalpel into the skin in front of the fetlock joint. Into this 'nick' the beak of Robert's fetotomy knife is inserted, and a long incision is made up the front of the limb from pastern to the scapular cartilage. The second step is literally the 'skinning' of the limb. This operation requires strong fingers, but with diligent application it may be completed in about 10 minutes. The third step is division of the adductor muscles by Robert's knife. The muscle mass is separated into several 'strings'; then each of these, in turn, is engaged and severed by the knife. The forth step is to disarticulate the fetlock joint so that the digit is left connected to detachment skin of the metacarpus. A snare is then attached to the cannon bone. The final step consists in an avulsion of denuded forelimb by the forcible traction (with traction bars and two assistants), while the operator applies counterforce to the front of the fetus. Should delivery not be possible after this operation, the other foreleg must be removed in the same way. Percutaneous fetotomy: This performed by fetotom (embrytom). The first operation is the removal of fetal head, neck and one forelimb. To do this the fetotom wire must be looped around the neck and forelimb and pushed back on one side so as to lie behind the position angle of the scapula where a deep incision is made with Unsworth's knife to accommodate the wire. The head of the fetotom is brought up to the base of the neck on the side opposite to the foreleg being removed (use the maximum length of available wire). The detached segment of fetus is carefully drawn out of the birth canal then an attempt is made to deliver the remainder of the calf by traction; a snare is placed on the intact limb and, with the aid of the double hook. If birth is not yet possible, the calf is repelled and the 21 wire is looped around the trunk with the head of the fetotom laterally. Sawing is continued until the vertebral column is severed. The remainder of the abdomen is eviscerated, the next step the wire is passed over the dorsal aspect of the sacrum and down behind the perineum, where the hand, passed in under the calf, reaches it, pulls it out and complete the loop. The hindquarters are divided by direct sawing; then each of the halves can be withdrawn by double hook. In comparing the facility between the S/C or percutaneous fetotomy, it must be clearly that the troublesome part of the percutaneous method is the correct placing and retention of the wire. Occasionally the two methods may be used combined. Fetomaternal disproportion: Posterior presentation: Vaginal delivery: The hind feet are usually visible at the vulva and to them snares are applied above the fetlock joints, in delayed cases fetal fluid supplements are essential. With one leg repelled as far as possible, the other is pulled on so as to bring its stifle over the pelvic brim. The repelled limb is similar deal with. In this way a smaller fetal diameter is presented at the pelvic inlet and traction may be success. If traction has not succeeded, the fetus must be removed by C.S or by fetotomy in dead fetus. Subcutaneous fetotomy: Posterior epidural anaesthesia is induced and a 'nick' made just above the fetlock on the posterior aspect of the external leg. Incision is made by Rober's knife from the fetlock to the anterior gluteal region. The skin is separated all around the leg and the muscles above the hip joint, as well as the adductor muscles are divided. The femoral head is detached from the acetabulum by traction bar introducing under the Achilles and by forcible rotating the limb laterally. The skin is then cut sufficiently around the fetlock joint to give scope for disarticulation, and a rope snare is placed over the end of the metatarsus. Traction should be applied by two assistants with retropulsion of the calf by the obstetrician, usually causing avulsion of the denuded limb. Then removal of this leg and other by traction and this result in extraction of the calf. Percutaneous fetotomy: In this technique, the wire loop placed over one foot and passed up the limb so laterally it lies anterior to the external angle of the ilium where a cut in the skin, previously made with Unsworth's knife. The head of the fetotom is placed lateral to the anus, and the tail of the calf must be included in the loop. The severed limb is removed. Traction is applied to calf by the Krey Schottler's hook attached to perineum (or aid by Obermayer's anal hook).If delivery still impossible, the other hindleg must be removed. Treatment of Dystocia due to postural defects Postural defect of anterior presentation: In cattle: Postural defects are a frequent cause of dystocia in ruminant species includes carpal flexion and lateral deviation of the head. Generally, postural defects are readily rectified by manipulation if treated early in 2 nd stage of labour. But in neglected cases associated with secondary uterine inertia, loss of fetal fluids and a dead, emphysematous fetus, very serious dystocia may occur, for which fetotomy or a C.S may be required. After the posture has been corrected, the cow must be delivered by traction. 1- Carpal flexion posture: One or both forelimbs may be affected. In the unilateral case the flexed carpus is engaged at the pelvic inlet; the other forefoot may be visible at the vulva. The simple 22 recent case requires retropulsion at the fetal head or shoulder; the retained foot is then grasped and, as the carpus is pushed upwards, the foot is carried outwards and finally brought forwards and extended. More difficult cases require a snare attached to the retained fetlock to help extend the limb. The fetal foot should always be carried over the pelvic brim in the cupped hand of the obstetrician. 2- Shoulder flexion posture: complete retention of the forelimb: This type of dystocia may be unilateral or bilateral. The diagnosis of bilateral retention is usually obvious by observing that the head partly or completely protrudes from the vulva, but in absence of the forelimbs. Retropulsion is very necessary, and if the protruded head is very swollen and the calf is dead, it should be amputated outside the vulva and traction may be applied by Krey’s hooks. Or the fetus is repelled, the retained forelimbs tend to come forwards; the calf’s radius and ulna are then grasped and the defect is easily converted into carpal flexion posture and relieved accordingly. In the more difficult case the limb must be snared. In delayed case, percutaneous fetotomy is performed (retained forelimb). 3- Lateral deviation of the head: When treated in early 2nd stage labour, it is easily corrected by hand, without recourse to epidural anaesthesia. The lubricated hand is introduced and, the provoked straining has ceased, the fetus is repelled by pressing forwards at the base of its neck. The hand is then quickly transferred to muzzle of the calf, which is firmly grasped and brought round through an arc until the nose is in line with the birth canal. In more protracted cases of dystocia due to head displacement, with greater loss of fetal fluid and with uterus contracted on the calf, it is more difficult to rectify the posture. Caudal epidural anaesthesia is indicated followed by the much of lubricant. A special head cord, of smaller caliber is placed over the mandible of the calf, where it is tightened, and the shank of the snare is handed to an assistant. The operator reintroduces a hand, grasps the calf’s muzzle and, as it is manipulated to extend the neck, the assistant is directed to apply gentle traction. In very neglected cases of dystocia when the fetus is dead with congenital rigid curvature of the neck (wryneck), correction is impossible and decapitation is required. (Wire-saw fetotom). 4- Downward displacement of the head: This uncommon type of dystocia in cattle. The more severe varieties of downward deviation of the head, namely ‘nape presentation and ‘breast head’ posture in which the head is flexed vertically between the forelimbs (rare in cattle); when presented, they have usually been caused by traction on the limbs before the head extended. Provided sufficient retropulsion can be achieved, vertex posture is easily overcome. Neglected cases may require caudal epidural anaesthesia and fetal fluid supplement. In very difficult cases, it may be advantageous to replace both forelimbs into the uterus. Casting the cow and placing her in dorsal recumbency may greatly facilitate extension of the fetal head. Postural Defects of anterior presentation in horses: Although showing a lower incidence in horses than in cattle, defects of limb posture cause more serious dystocia in mares than in cows. This due to the severe pelvic impaction that is consequent upon the mare's very strong expulsive efforts and to the longer limbs of foals. In order to prevent rupture of the uterus or vagina, correction of posture must be done very carefully. 1- Carpal flexion posture: The principles of correction are the same as for the cow. There is a tendency for a foal in carpal flexion posture to become impacted in the maternal pelvis; the 23 procedure required will depend on the degree of impaction, on the relative sizes of the fetus and birth canal and on the duration of 2nd stage labour. Retropulsion of the fetus, followed by extension of the carpus, should always be attempted. If there is obviously insufficient room for extension, the flexed carpus may be pushed forwards into the uterus so that retained limb lie under the fetal abdomen. Moderate traction applied to the other limb and to the fetal head then often succeeds without injury to the mare. If it is impossible to relieve the impaction, attempt traction without correction (with snares) or section the leg through the carpal joint. 2- Shoulder flexion posture: One or both forelimbs may be retained. Copious fetal fluid supplement should be infused and vigorous retropulsion applied. Once the radius and ulna have been snared it should be possible to advance the limb and to convert the posture into one of carpal flexion and then to proceed accordingly. When both forelimbs are retained and attempts at correction fail, fetotomy should be applied. 3- Foot-nape posture: It is upward displacement of one or both extended forelimbs so that they come to lie above the extended head in the vagina. It is a postural defect peculiar to the horse that is made possible by the more slender head and longer limbs of the foal. The foal's muzzle is vigorously repelled in a cranial and upward direction the fetal foot is raised and then pushed or pulled, to the correct side. The other foot is similary manipulated and, finally, the head is again raised and each forelimb placed underneath it. Traction is then applied to the head and both limbs. If penetration of vaginal roof has occurred, epidural or general anaesthesia should be induced. Reposition is first attempted and if it is impossible, amputation of the head or upper limb should be performed. 4- Lateral deviation of the head: This is a more serious malposture in horses than cattle because, owing to the greater length of the neck and head. Special instruments are required to access the head such as Kuhn's crutch, Blanchard's long flexible hook and Krey- Schottler double hook. In cases of 'wryneck', the head and neck must be amputated by wire-saw, or a C.S performed. 5- Downward deviation of the head: The methods of correction are the same as for the cow. Extension of the head requires the application of a mandibular snare, and while firm pressure is placed upon the fetal brow with one hand, the snare is pulled upwards and backwards by an assistant. In obstinate cases of nape posture with impaction at the pelvic brim, fetotomy is indicated. Postural Defects of posterior presentation: It is more difficult to correct than the anterior defects, particularly in horses. There are three essential requirements in attempting to correct the posterior defects: namely, epidural anaesthesia, fetal fluid supplementation and retropulsion. All manipulations should be conducted very carefully and gently to avoid accidental perfusion of the uterus. 1- Hock flexion posture: In the cattle, the condition is usually bilateral. The points of the hock may be felt in front of pelvic brim. In early cases, with or without epidural anaesthesia, the posture may be corrected by hand. The fetus is first repelled by pressing forward in its perineum, and the hand then grasps the fetal foot and drawn it back through an arc. In cases which it is found to be impossible to extended the hock due to lack of space, the point of the hock is press forwards and upwards by hand of assistant while the operator 24 applied traction by snare fixed to the retained foot around the pastern and between the digits. When the calf is dead, simple fetotomy may be performed. In the horse, the correction is very difficult due to longer limbs of the foal. Fetotomy or a C.S will be required. 2- Hip flexion posture: In the cattle, when both hind legs are retained in the uterus, the case is described as 'breech presentation'. Usually on vaginal examination, the calf's tail is recognized. The aim of the treatment is to convert the condition into one of hock flexion posture and then proceed accordingly. The need for epidural anaesthesia and fetal fluid supplement will be primary considerations. The manipulative procedure is to repel the calf's perineum forwards and upwards with a view to bringing the retained limbs within reach, when the may be grasped as near to the hock as possible, traction on the limb converts the posture into hock flexion and proceed accordingly. If it's impossible to bring the hock within reach, and the calf is dead, then fetotomy may be performed. In the horses, sometimes a mare will foal unaided despite complete retention of the hind legs. However when dystocia is occurred, a C.S or fetotomy should be performed under general anaesthesia. Treatment of dystocia due to defects of presentation or position a) Position: Faulty position of the fetus is encountered more frequently in horses than in cattle. This due to failure of physiological rotation of the fetus from the ventral to the dorsal position. 1- Anterior presentation, lateral position: In the case of a live calf or foal, the obstetrician passes his hand to the fetal head and by the thumb and middle finger, presses on the fetal eyeballs to causes a convulsion reflex response in the fetus and, by applying a rotational force in the appropriate direction, it is easy to turn the fetus into the dorsal position. Should this method fail, then snares are attached to the limbs and caudal epidural anaesthesia is induced; rotation is performed mechanically, firstly by repelling it as far cranially as possible, crossing the snares in the appropriate direction, and then by applying traction. 2- Anterior presentation, ventral position: The correction is similar to that as described for lateral position defect, although the procedures will usually need to be repeated several times. 3- Posterior presentation, lateral position: The operator introduces a hand and grasps the stifle region of the upper limb. Simultaneous retropulsion and downward pressure are applied to rotate the fetus through 90º. 4- Posterior presentation, ventral position: The operator introduces a hand between the fetal hindlimbs and up to the inguinal region, where one of the thigh is grasped; then pushing forwards, the operator rotates the fetus through a half-circle. Failing this, traction on crossed limb snares should be used. The hind feet of a foal in ventral position will penetrate the vagina and rectum. In such a case a C.S should be performed and the rectovaginal fistula repaired later. 25 b) Presentation: 1- Oblique dorsovertical presentation: According to whether the head or breech is nearer the pelvic inlet, the presentation is converted into anterior or posterior longitudinal. An attempt is made is to bring the fetal extremity (head or limbs) to pelvic inlet, and firstly to convert the defect into a ventral longitudinal presentation. The fetus can be then rotate to the dorsal position. While retropulsion is applied, the Krey’s hook is pulled on with a view to bringing the fore or hind end of the fetus to the pelvic inlet. After adjustment of position and posture, the fetus is then delivered by gentle traction. Failing this version, a C.S performed. 2- Oblique ventrovertical presentation (dog-sitting position): It is still rare and is only likely to be encountered in the mare. In this defect the foal being disposed with its fore end advanced to a variable degree in the vagina and it is hind parts in the uterus. It differs from normal anterior presentation in that the hindfeet also pass into the birth canal and rest on the pelvic brim. Most cases are severely impacted, but after the induction of epidural anaesthesia and the infusion of lubricant fluid into the uterus, an attempt should always be made to repel the fetus sufficiently to allow the hind feet to be pushed off to pelvic brim into the uterus and thus to convert the dystocia into simple anterior presentation. Traction is then applied. Placing the cow or mare on dorsal recumbency with hindquarters elevated often helps. Should this attempt fail, then a C.S is the effective method of treatment. 3- Dorsotransverse presentation: This is a rare cause of dystocia. The technique of correction required involves repulsion of the fetus, and the advancement of its nearer extremity to the birth canal. The cow should be given an epidural anaesthetic, and in the mare general anaesthesia should be induced. Fetal fluid supplement should be applied and an attempt made by manipulation of the proximal fetal extremity to turn the fetus into ventral position, anterior or posterior presentation. The next step is to rotate the fetus into dorsal position. Finally, it is delivered by traction. If after a short determined effort it is obvious that version cannot be achieved, a caesarean operation should be performed immediately. Fetotomy is very difficult to carry out in this type of dystocia and consequently is not recommended. 4- Ventro-transverse presentation: This presentation is more likely to be seen in the mare than in the cow. A variable number of fetal appendages may enter the maternal pelvis. It is usual for two or more legs only to be presented. The condition must be distinguished from twins and double monsters and from schistosoma reflexus. The aim of vaginal interference is firstly to convert it into longitudinal-posterior-presentation, ventral position; this means that the posterior extremity must be advanced while the anterior extremity is repelled. General anaesthesia and dorsal recumbency are helpful in the mare. In the bicornual type of transverse presentation (in the mares) the fetal extremities are disposed in the two horns and it is trunk lies across the anterior position of the uterine body. Ventral displacement of the uterus may have occurred (it may impossible to palpate the fetus). This presentation is required a C.S. Dystocia due to Twins: Twin gestation in cattle often causes dystocia, but in mare’s abortion is a more likely sequel. In sheep, may cause dystocia due to mal-disposition. Twin dystocia is three types: 26 Both fetuses present simultaneously and become impacted in the maternal pelvis. One fetus only is presented but cannot be born because of defective posture, position or presentation; posture is often most at fault, the lack of extension of limbs or head being due to insufficient uterine space. In uterine inertia, defective uterine contractions are caused, either by overstretching of the uterus by the excessive fetal load, or by premature birth. In the treatment of twin dystocia, the first essential is diagnosis. It is very important, that the fetal appendage is identified. The obstetrician will not blunder into applying traction simultaneously to two fetuses. Nor should twins be mistaken for monsters or ventrotransverse presentation of a single fetus. Where a twin is presented with an abnormality of posture, it is treated as if it were a single fetus; in such cases the presence of twins is not known- but may be suspected on account of small fetal size and the history of the dam- until the uterus is searched after delivery and another fetus found. Simultaneous presentation would seem probable when a twin from each horn approached the pelvic inlet; abnormality of posture and inertia would be more likely when both fetuses occupied the same horn. When twins are known to be present and retropulsion is required- either of the presenting fetus to correct its posture or of the less advanced to allow delivery of the first twin it should be performed very carefully. Simultaneous presentation of twins is treated in logical sequence. The polarity of the fetuses is determined, the more advanced fetus recognized and its presenting extremity snared. Any defect of presentation, position or posture must be diagnosed and treated; correction may be greatly facilitated by epidural anaesthesia. Then with continuing retropulsion on the less advanced fetus, the first one is brought into the pelvis and delivered by simple traction. The other fetus, which may be presented in the opposite direction, is then manipulated. If corrective manipulation is impossible, fetotomy of the presenting fetus may be required or a C.S should be performed. Most cases of equine twin conception are followed by early death of one or both of the conceptuses. Dystocia due to Monstrosities: Monstrosities most often cause dystocia in dairy cow, the commonest example being schistosoma reflexus, persomus elumbis, double monsters, Achondroplasia and dropsical fetuses. Monstrosities are uncommon in mares (with the exception of wryneck). With the exception of anasarcous fetuses and gross malformation is often associated with ankylosis of joints, many monster weight less than normal calves and may be sufficiently small to be passed spontaneously. Principles of the delivery of monstrosities: Recognition of the exact disposition of the fetal extremities, and an estimate of fetal size, may be very difficult. Careful traction- with due regard to lubrication and protection of the birth canal from irregularly disposed appendages may be applied. Prior to the attempt at vaginal delivery, the diameter of dropsical fetuses may be reduced by multiple or single incisions with fetotomy knife. If moderate traction does not succeed, fetotomy or a C.S must be performed. Obstetric management of schistosoma reflexus: This most familiar bovine monstrosity. The weight of the monster calf is usually around 22 kg. It may be presented viscerally or by its extremities. With this type of dystocia, fetal viscera may be seen protruded from the vulva (or by vaginal exploration). The viscera may be mistaken for those of the mother and uterine rupture may be 27 suspected. The viscera must be torn away from the fetus whose rigid vertebral angulation may then be felt at the pelvic brim. Reasonable traction, with adequate lubrication is now applied (with Krey's hooks). Where, after a short period of traction, it is obvious that safe vaginal delivery is not possible; the fetus should be bisected by the wire-saw fetotomy. When a schistosome presents by its extremities- three or four legs, with head, fetotomy or a C.S will be required (C.S may be prefer). After removal of a schistosome, the uterus may be searched for injury. 28 Injuries and diseases incidental to parturition 1- Contusions and laceration of the birth canal: Any part of the birth canal may suffer contusion during forcible extraction of the fetus, but the cervix and vulva are more likely to be lacerated than the dilatable vagina. All vaginal contusions and lacerations should be treated with mild emollient and antibiotic. Laceration of the cervix may be sutured by applying Vulsellum retraction forceps to the organ and with drawing it to the vulva. Wounds of the vulva and perineum are easily sutured. If lacerations of the vulva and perineum are not sutured, scar tissue formation and distortion impede the sphincter action of the vulva, with consequent aspiration of air, vaginitis and metritis. i. Haematoma of the vulva: This is a sequel to contusion of the sub mucous tissue during delivery. One lip of the vulva is usually affected and round swelling occupies the vulva orifice. It may be confused with prolapse, tumour or cyst of vagina. If left untreated, natural resolution usually occurs within a few weeks. ii. Perineal injuries at parturition: Serious perineal injuries occur during the 2 nd stage of labour in both the cow and the mare. These injuries may be classified to: a) First degree: tears of vulvar lips only (skin and mucous membranes). The repair involves; episioplasty (Caslik suture) to prevent pneumovagina, application of antibiotics inside the uterus and injection of tetanic antitoxin (TAT) are recommended (in the mare). b) Second degree: tears at the vulvo-vaginal sphincter including connective tissue and muscles. The repair is preceded by a time gap (about 6 weeks) to allow healing by granulation. It involves surgical reconstruction and repair of the perineal body, vulvar lips and sphincter by the same procedure adopted for Caslik surgery. In addition to urethral extension to correct urine pooling or pneumovagina. Application of antibiotics inside the uterus and injection of tetanic antitoxin (TAT) are recommended. c) Third degree: cloaca formation from a tear extending through the rectovaginal shelf, perineal body and sphincter, and vulvar lips. The repair of the 3rd degree is preceded by a time gap (6 weeks) to allow healing by granulation and application of antibiotics inside the uterus and injection of tetanic antitoxin (TAT) are performed before repair. d) Fourth degree: rectovaginal fistula forming communication between the rectum and vagina without disruption of perineal body, anal sphincter or vulvar lips. In the cow the perineal tearing due to vulval stretching, while in the mare it is essentially from the perforation of the vaginal roof by a fetal forelimb. 2- Rupture of the uterus or vagina: Uterine rupture may occur spontaneously, but faulty obstetric technique is a more frequent cause. Spontaneous rupture may associate with uterine torsion or with cervical non-dilatation but is also possibly due to the gross uterine distension that occurs with twins in one horn, with hydrallantois or with excessive fetal size (rupture occurs in late gestation or during labour). When rupture occurs during labour and the fetus passes into the abdomen, labour pains and straining cease and uterine inertia may be suspected. Alternatively the dam’s intestine may prolapse into the uterus and even protrude from the vulva (this condition may confuse with dystocia due to schistosoma reflexus in visceral presentation). Accidental rupture of the uterus is occurs due to: 29 Insufficient uterine space for the extension of a limb or head. Inordinate traction on a wrongly disposed or oversized fetus. Excessively vigorous retropulsion. Rupture of the uterus may be due to external violence (falls heavily or severe kick or horn-gore on its abdomen). When the uterine rupture is discovered, laparotomy is indicated. Spontaneous rupture of the vaginal wall is common in late pregnant ewes. Small intestine passes into the vagina and protrudes from the vulva, frequently the ewe will be found dead (from shock). The precise aetiology of disorder is still unknown; it is generally believed to be associated with cervical vaginal prolapse. 3- Prolapse or eversion of the bladder: Prolapse of the bladder may follow a rupture in the floor of the vagina or eversion through the dilated urethra and may occur during or after parturition. The condition must be differentiated from prolapse of the vagina, cyst or tumour of the vagina and haematoma of the vulva. The surface of bladder is cleaned and punctured with a hypodermic needle then dressed with an antibiotic powder and gently pushed back into place through the vaginal rupture. Eversion of the bladder is most likely in the mare due to the urethral opening is wide and parturient straining very forceful. The organ becomes everted during labour and may be injured during fetal expulsion. Epidural anaesthesia should be induced. The bladder is cleaned, and any lacerations are repaired by suture. The organ is then compressed between both hands and gradually forced back into the urethra. Further manipulation is then applied to the vaginal floor until the bladder is properly replaced. Antibiotic and tetanus antitoxin should be given. Eversion of the bladder is rare in cattle. 4- Prolapse of the rectum: Slight eversion of the rectum is a common accompaniment of powerful expulsive efforts. It recedes after delivery. Severe prolapse has been present for some hours, the organ has become oedematous and contused or torn, and it may be difficult or impossible to replace it. Submucous resection under general anaesthesia must then be applied. Parturient prolapse of the rectum may be fatal because stretching or tearing of the colic mesentery can result in infarction of the terminal colon. 5- Parturient recumbency: It is occasionally seen in all species but is essentially a bovine condition. Cows which become recumbent in late gestation should be first being considered due to poor nutrition (starvation). A prompt C.S and dietary supplementation are indicated. Hypocalcaemia is the cause of recumbency in parturient and puerperal cows. Puerperal metritis usually follows dystocia and is accompanied by retention of placenta may cause recumbency. Other severe toxaemias that cause parturient recumbency are acute mastitis, traumatic pericarditis and peritonitis associated with uterine rupture. Physical inability to rise due to muscular weakness or to lesions of the locomotors system (dislocation of hip joint, pelvic fracture and paralysis of obturator or gluteal nerves) are cause recumbency. Vaginal prolapse: It is protrusion of the floor, lateral sides and portion of the roof of vagina through the vulva. If the cervix is involved it is then called vagino-cervical prolapse. Cervicovaginal prolapse (CVP) is more common in stabled than in pastured animals, suggesting that lack of exercise may be a contributing factor. 30 The condition can be common in late gestation of cattle, camels, sheep and goats (may occur after parturition) for the following reasons: a) Increased intra-abdominal pressure associated with increased size of the pregnant uterus, intra-abdominal fat and perivaginal fat or rumen distention. Intra-abdominal pressure is increased in recumbent animals. Added to this, sheep tend to face uphill when lying down, so that gravity assists vaginal eversion and prolapse. b) Increased oestrogenic activity prepartum. c) Animals grazing on (or supply) high oestrogen-like substances, such as barley, moudly cereals, clover or maize (especially Trifolium subterraneum). d) Inadequate exercise. e) Severe straining in response of vaginal trauma, or infection, following serious dystocia. f) Inherited factor. g) Vaginal prolapse may also be a problem in cows subjected to repeated superovulation for embryo recovery. h) A genetic component in the pathogenesis of cervico-vaginal prolapse is likely because a breed predisposition exists in both cattle (Brahman, Brahman crossbreds, Hereford, Simmentals and Charolais) and sheep (Kerry Hill, Romney Marsh). It is uncommon in pregnant horses and pigs. The symptoms of vaginal prolapse vary from mild prolapse, when the vagina only protrudes with the sitting position of the animal, but recedes when the animal stands up, to serious vaginal prolapse, that the vagina is always protruding. Long standing cases show congestion and ulcerations of the everted part and animal could by anorexic with rapid deterioration of health and death may follow. Treatment of vaginal prolapse is as follow: 1) Cessation of straining by giving an epidural analgesia. 2) Gentle and thorough cleaning of the everted part by warm antiseptic solution to enhance blood circulation. 3) Application of an oily antibiotic in order to kill germs and facilitate reduction of the everted part. 4) Gentle reduction of the everted vagina by the vest hand or a clean towel until the vagina is in situation. 5) Retention is achieved by insertion of a Buhner suture—a deeply buried, circumferential suture (a purse string suturing using a subcutaneous needle and cotton tape) placed around the vestibulum to provide support at the point at which the initial eversion of the vaginal wall occurs. Buhner sutures should generally be removed before parturition to prevent extensive laceration. 6) Care should be taken to let a passage for urine. The use of prolapse retainer, composed of plastic nylon strapping, fitted around the perineal region can be helpful for retaining the prolapsed vagina until the announcement of parturition. Post parturient prolapse of the uterus: Prolapse of the uterus is eversion of uterus to the outside, often with placenta still attached and one or both uterine horns are hanging behind the animal. It's a common complication of the 3rd stage of labour in the cow and the ewe and is rare in the mare and bitch. In the cow, multigravidas are more affected than are heifers. 31 Aetiology: The cause of uterine prolapse is not clear, but there is no doubt that it occurs during the 3rd stage of labour, within a few hours of the expulsion of the calf, and at a time when some of the fetal cotyledons have separated from the maternal caruncles. Underlying causes: 1. Uterine inertia. The eversion and prolapse are associated with onset of uterine inertia during 3rd stage of labour (straining pushes the flaccid uterus) when a portion of detached afterbirth occupies the birth canal and protrudes from the vulva. 2. Increased straining caused by pain and discomfort after parturition. 3. Intra-abdominal pressure including tympany and recumbency. 4. Excessive traction at assisted parturition. 5. Retained fetal membranes. 6. Some cattle with extreme laxity of the perineum and vulva ma prolapse immediately after every calving. Prognosis: The prognosis will depend on: a) The type of the condition. b) The duration of the case. c) The organ has sustained severe injury. The condition occurs after normal parturition, and professional assistance is forthcoming within an hour or two of its occurrence, the prognosis is good. Occasionally uterine prolapse is followed by the animal's death. On post-mortem examination in such cases it is found that death was due to internal haemorrhage caused by torn the mesovarium and ovarian artery. Treatment: 1- Replacement of the everted organ: If the cow is recumbent, the farmer should be instructed to wrap the prolapsed uterus in a large towel to prevent further contamination; if she is standing, the organ should be supported by a large towel or sheet held by people on either side, until professional assistance is forthcoming. It is good to give a preliminary injection of calcium borogluconate and to relieve ruminal tympany, if present. Epidural anaesthesia should be given to prevent straining and abeyance of defaecation during the operation. The everted organ should be washed with warm normal saline solution. If the fetal membranes are already partially detached, can be removed without injury to the caruncles. But when attachment is complete, it is better that the organ be replaced with membranes. The uterus should supported by assistants holding the corners of towel. The operator commences to replace the uterus little by little, starting with those portions nearest the vulval lips. It is generally best to replace portions of the upper and lower surfaces alternatively. Oxytocin should be given to restore uterine tone and thus to prevent recurrence of the prolapse. Parentral antibiotic and non steroidal anti-inflammatory agent should be given. Suture of the vulva to prevent the possibility of re-prolapse. 32 2- Amputation of the everted organ: This operation can be adopted, when the uterus is severe damaged and replacement of organ is impossible. 33 Infertility in female farm animals Infertility in the cow: Infertility means delayed or irregular production of the annual live calf (Infertile: Incapable of initiating, sustaining, or supporting reproduction. Alternatively, not fertilized and therefore incapable of growing and developing.). Subfertility means diminished reproductive capacity or less than normal capacity for reproduction.Sterility is used to mean an absolute inability to reproduce. Both congenital and acquired abnormalities of the genital system can influence fertility. Lesions of the ovaries: 1- Congenital lesion of the ovaries: Congenital lesions of the ovaries are rare. A few reports show one or both ovaries are absent (ovarian agenesis), accompanied by an infantile genital tract and absence of cyclical behaviour. Ovarian hypoplasia is a little more common, in this condition; one or both ovaries are small, functionless and composed of largely undifferentiated parenchyma. Oocytes and follicles are virtually absent. 2- Acquired lesions of the ovaries: The most common of the acquired lesions of the ovaries, cystic ovarian disease. Ovaritis (oophoritis) is very rare lesion of the ovary. Granulosa cell tumours and fibromas are generally the most common neoplasms of the bovine ovary. Granulosa cell tumours can produce any of the main ovarian steroids (oestrogen or androgen are common). Tumours that secrete oestrogen cause animals to display nymphomaniacal behaviour. Androgen secreting tumours are more commonly associated with anoestrus. Other tumours of the bovine ovary include carcinomas and sarcomas. These tumours are generally benign and massive. Abnormalities of the uterine tubes, uterus and cervix: 1- Segmental aplasia of the paramesonephric ducts (Mullerian): Development defects of the Mullerian ducts lead to a wide range of anomalies of the vagina, cervix and uterus. Depending upon the site of aplasia, the cow may be sub fertile or sterile. However, the ovaries develop normally (normal cyclic behavour). In some cases, the whole of the vagina, cervix and uterine horns may lack patency. More commonly, partial or segmental aplasia of the paramesonepheic ducts occurs. In the case of uterus unicornis, only uterine horn has a lumen, the other appearing as a narrow, flat band (right horn to be absent more than the left). Provided the remainder of the genital apparatus is normal, individuals may conceive to ovulations from the sound side. A more serious type of aplasia occurs when isolated sections of uterine horn are present. Uterine secretion accumulates and cause sac-like dilatation of such isolated portions of the tract (this may confused with early pregnancy). Animals with this deformity are sterile. Abnormalities of the cervix may include duplication of its lumen; each uterine horn connects with the vagina by a separate cervical canal (uterus didelphys). Affected animals conceive normally (dystocia may show due to fetal limbs entering each cervical canal). A similar complication may arise in heifers with a single cervix opening into a double os uteri externum. 34 In the cases of uterus didelphys, a double cervix is present, the uterine body is divided. Failure of fusion is complete of both paramesonepheric ducts. The most common developmental defect of the female tubular organs involves a variable degree of persistence the hymen. This may appear as vaginal constriction in front of the urethral opening (discovered at parturition), as a partion with a central aperture or as complete partition between the vulva and vagina. The second and third types are discovered when investigating heifers which either strain forcible after service, or cannot be inseminated artificially. Where hymenal obstruction is complete, there is an accumulation of secretions in front of the obstruction which cases a fluctuating swelling may be palpated per rectum. The hymenal defects occur commonly in white shorthorn heifers and described as ‘white heifer disease’. This condition is considered to be due to a sex-linked recessive gene with linkage to the gene for white coat colour. 2- Freemartinism: Freemartin is heifer which is born co-twin to bull and is a distinct form of intersexuality which arises as a result of a vascular anastomosis of the adjacent chrioallantoic sacs of heterozygous fetuses in multiple pregnancies (occur as early as 30 days of gestation) (cellular theory: migration of male cells to female cells prevent the growth of female cells and converted to male cells-like. Hormonal theory: male genitalia are more growing than female genitalia due to mullerian duct inhibiting substance factor). As a result, although the external genitalia of freemartin heifers appear normal, the internal genitalia are grossly abnormal. The structures derived from the paramesonephric ducts are almost entirely absent or are grossly hypoplastic. In animals that have undergone a significant degree of masculinization, the gonads resemble testes, to extent that their parenchyma contains recognizable tubules and interstitial tissue. It is generally assumed that 95% of heifers which are born as co-twins to bull are sterile freemartins (with sex chromosome XX/ XY chimerism. A chimera is an animal that has two or more different populations of genetically distinct cells that originated in different zygotes involved with sexual reproduction. Diagnosis of freemartin: The newborn freemartin may be recognized by it is prominent clitoris with obvious tuft of hair at the inferior of the vulva (this signs are not always reliable). In the adult, the length of the vagina is less than (normal: 30 cm, abnormal: 810cm.) and rectal palpation will fail to identify the cervix. Fenscher’s pencil test in calves of 1-4weeks of age (normal length: 13-15 cm, abnormal: 5-6 cm) can be made using pencil or blunt probe which should be inserted to vagina (not introduced). Chromosomal analysis at any time after birth by take blood sample and cultured to demonstration of sex chromosome chimerism. More recently, PCR for presence of Y chromosoes in blood cells has been described. 3- Hermaphroditism: It means the animals which have a primary sex organ of male and female and a secondary sex organ of one or both. There are two type of hermaphroditism: a- Physiological hermaphroditism: Such as in the flowers, some worms like Tenia.spp, fishes and snails. b- Pathological hermaphroditism: It may be occur in all animals, but is more common in the goat without horn (recessive gene). It may be classified to: 35 (i) True hermaphroditism: Refer to present both of the primary sex organs of male and female and it may be bilateral (have testes and ovary in each side), unilateral (have testes and ovary in one side and testes or ovary in the other side) or lateral (have testes in side and ovary in the other side). Generally the true hermaphorditism is rare. (ii) Pseudo hermaphroditism: Refer to present of the primary sex organs only of one gender with the secondary sex organs of the other. It may be male pseudo hermaphrodite (have testes and female secondary organs) or female pseudo hermaphrodite (ovary and male secondary organs). For avoidance of hermaphroditism, mating in horns should be applied. Metritis complex: It is common for terms metritis, endometritis and pyometra. These diseases share common etiological factors, predispose to one another and, to a large extent, share common treatments. Under normal circumstances, there are several mechanisms which prevent opportunist pathogens from colonizing the genital tract. I. The uterus is protected by the physical barriers of the vulval sphincter and cervix. II. The uterus is protected by local and systemic defense mechanisms; both are influenced by the reproductive steroid hormones, oestrogen and progesterone. Puerperal or peracute Metritis: It is inflammation of all layers of the uterine wall that develops within a few days to several weeks after parturition. It usually follows a severe dystocia and may be associated with uterine inertia, twin birth, RFM and damage to the vulva and/ or birth canal. The most important infecting organisms cause metritis is A.pyogenes, streptococci, haematic staphylococci and Gram-negative anaerobes (Bacteroides spp). Sypmtoms: Toxaemia, septocaemia and pyaemia. The temperature of affected cows may be elevated to 40-41C°, but is more often subnormal. There is a rapid pulse rate (100/min.) and respiratory rate. Anorexia, dehydration and diarrhoea (signs of shock). The uterus contains a large volume of toxic, fetid, reddish, serous exudates (the exudate is discharged from the vagina by frequent straining). The cotyledons are swollen and the fetal membranes often remain firmly attached. The vulva and vagina are swollen and deeply congested. Puerperal metritis must be differentiated from pneumonia, traumatic pericarditis and reticulitis, milk fever and acute mastitis. Treatment: o An attempt should be made to remove the fetal membranes by very gentle external traction without enter the vagina and uterus with the hand (to avoid severe damage and predispose to the absorption of toxins). o If the cow is continually straining, caudal epidural anaesthesia can be used. o If the case is seen within 2-3 days of parturition, 50 I.U of oxytocin by I/V injection may cause contraction of the uterus and expulsion of fluid and debris. o Administration of antibiotic (parenteral), supportive therapy and anti-inflammatory drugs. 36 Prognosis: The prognosis for subsequent fertility should always be guarded (if perimetritis or parametritis is present, it is relatively poor), since cows that have suffered a severe puerperal metritis very often develop lesions such as ovarobursal adhesions, uterine adhesions and occluded uterine tubes. Other complications of metritis include pneumonia, arthritis and endocarditis. Endometritis: This implies inflammation of the endometrium. Unlike metritis, it does not affect the general health of the cow. Causes of endometritis: The great majority of cows suffer from bacterial contamination of the uterus after calving, but, under normal circumstances, this flora is rapidly eliminated. When the bacterial flora is not eliminated from the uterus, causing the endometritis. The pathogensis of disease is largely concerned with factors that impair the ability of cow to eliminate the infection, rather than with bacteria themselves. There are many factors that are associated with the development of endometritis: a) Retained fetal membranes and conditions that lead to RFM are also associated with development of endometritis. These include: multiple births, abortion and induced calving. b) Dystocia is predispose to endometritis for several reasons: High incidence of RFM than normal calving. Damage to maternal tissues causing devitalisation. The obstetrical interventions to correct the dystocia increase the load of pathogens within the uterus. c) Management factors: High milk yield is associated with an increased incidence of endometritis. Overfeeding is associated with endometritis, particularly where the animals develop ketosis and fatty liver syndrome. Conversely, underfeeding has also been associated with endometritis (hypocalcaemia). Season of the year (cows calving during the winter or spring is more prone to endometritis than those calving at other time). d) Return to cyclical ovarian activity: In cows that ovulated early (first ovulation was 15.5 days), the bacterial contamination was not eliminated at the oestrus, so that when a luteal phase followed the bacteria were able to proliferate and colonise the uterus. e) Bacterial loading. In a dirty, unhygienic calving environment predisposes to the disease. Clinical signs: Endometritis may be clinical or subclinical. Clinical endometritis is characterized by: 1. Presence of a white or whitish-yellow muco-purulent vaginal discharge (leucorrhoea) in the postpartum cow. 2. The cow rarely shows any signs of systemic illness. Subclinical endometritis is characterized by neutrophils in uterine luminal fluid, but without visible purulent material. Diagnosis: o The presence of endometritis can be determined by trans-rectal palpation of the uterus (poor involution with has doughy feel). Now this method is not sensitive and specific for accurate diagnosis of endometritis, because too many other factors interfere with the rate of change of size of the uterine horns over the postpartum period. 37 o Manual examination of the vagina and cervix for presence of purulent discharges. This method is no longer recommended, since it causes considerable discomfort to the cow and has low specificity. o Alternatively, a vaginoscope can be used to examine for the presence of discharge around the cervical os or cranial vagina. o The most recently developed method is a rubber diaphragm on the end of a stainless steel rod (Metricheck), which is inserted into the vagina to collect a sample of discharges from the external cervical os/ cranial vagina. o Collection of cervical swaps for examination for the presence of neutrophils is effective means of diagnosing chronic infection. o Uterine biopsy has been used to study both the incidence of clinical and subclinical endometritis. Treatment: I. In many cases of endometritis are self limiting and resolve after the resumption of oestrous cycle. II. Antibiotics that have been formulated for intrauterine use include oxytetracycline (as an infusion or in the form of pessaries) and cephapirin (as an infusion). III. In the case of A. pyogenes has become dominant, intrauterine infusions of penicillin are effective. IV. When there is a palpable mature C.L on the ovary, PGF2α or its synthetic analogues should be required. V. Administration of oestrogens I/M may be useful for increase uterine blood flow and stimulate changes that occur during follicular phase of oestrus cycle. Consequences: Endometritis reduces fertility by extending the calving to conception interval (C.C.I), increase the number of services per pregnancy and the proportion of cows that fail to conceive. Endometritis can cause long-term, irreversible changes to the genital tract, thus increase culling rate. Pyometra: Pyometra is characterized by a progressive accumulation of pus in the uterus and by the persistence of functional luteal tissue in the ovary. Causes: In most cases pyometra occurs as sequel to chronic endometritis. The CL of dioestrus persists and the genital tract remains under the influence of progesterone. Death of the fetus, invasion of the uterus by A. pyogenes and retention of the CL of pregnancy (infrequent cause). Venereal infection with Trichomonas.fetus which causes embryonic death also causes pyometra. Clinical Signs: Cows show few or no signs of ill health; the main reason is the absence of cyclical activity. The uterine horn are enlarged and distended. Differentiation of pyometra from a normal pregnancy can sometimes be difficult, but there are number of distinguishing points: The uterine wall is thicker than at pregnancy. 38 The uterus has a more ‘doughy’ and less vibrant feel. It is not possible to slip the allantochrion. In most cases of pyometra, no uterine caruncle can be palpated. However, when the infection occurred in a non-involuted uterus, involution of the caruncles is delayed and they may remain palpable for quite a long time. Transrectal ultrasonography will demonstrate the absence of a fetus (presence of a‘speckled’ echotexture of the uterine contents). Treatment: The best treatment is the use of PGF2α or its analogue. They result in regression of the CL, dilatation of the cervix and expulsion of purulent fluid, with oestrus occurring 3-5 days later. If there is any doubt about the diagnosis of pyometra the cow should left untreated and re-examined 2 weeks later for evidence of change. Retained fetal membranes (RFM): RFM is a recurrent theme in considerations of the metritis complex of diseases.It is a common complication of bovine parturition and important contributor to bovine infertility. Aetiology and pathogenesis: RFM occurs when the normal processes of dehiscence and expulsion fail to take place. There appear to be three main factors involved in the separation and expulsion of the fetal membranes: Maturation of the placenta: The main changes that occur during maturation of the placentome are: a) Flattening of the maternal crypt epithelium. b) Migration and increased activity of leucocytes. c) hyalinisation of the blood vessel walls in the placentome. d) Relaxation of the superficial layers of the cotyledon is induced by proteases and colagenase. e) changes in the composition of the ‘glue line’ adhesive proteins between the cotyledonary and caruncular epithelium. Exsanguinations of the fetal side of the placenta, when the umbilicus ruptures, which causes collapse and shrinkage of the trophectodermal villi and their physical separation from the maternal crypts. Uterine contractions, which aid in the exsanguinations of the fetal side of the placenta ant aid in the physical separation of the placenta by distorting the shape of the placentomes (unbuttoning of the cotyledon from the caruncle). Predisposing factors: 1. Premature calving is very commonly associated with RFM. Cattle twins are usually slightly premature; hence, their birth is often followed by retention. 2. Placentitis and RFM occur in cases of abortion due to B.abortus, Salmonella Dublin, Compylobacter fetus and Aspergilus. Enlargement of the placentomes, in the absence of placentitis, also lead to retention. 3. Dystocia, primary uterine inertia and delivery by C.S. 4. Deficiency of selenium and vitamin E (antioxidants) may cause failure of oestrogen synthesis in the placenta. 5. Abnormalities of oestrogen: progesterone ratio in late gestation. 6. Heat stress, which can result in shortened gestations. 7. Reduced immune response to the uterus (failure of the release of inflammatory mediators). 39 8. There is some evidence of hereditary predisposition to RFM. Beef breeds are much less affected than those dairy breeds and the incidence is higher in Ayrshires than Friesian. Old cows are more affected than young ones. Clinical Features: It should be noted that cows which fail to expel the fetal membranes within 36 hours. Myometrial contractions largely cease from 36 hours after the birth of calf, so, if the membranes have not been expelled by this time, freeing of the foetal villi from the maternal crypts eventually occurs as a result of autolysis and bacterial putrefaction. The duration of retention seems to depend on several factors, such as the extend of the areas of attachment of the fetal membranes, the rate of uterine involution, the amount of uterine exudates and the proportion of the afterbirth which had already passed through the cervix when retention began. The toxic products of putrefaction accumulate within the uterus causing a fetid odour which pervades the atmosphere and taints the milk. Delayed uterine involution and a variable degree of metritis commonly accompany by retention. Influence of RFM upon fertility depends on the develop metritis. Yet for majority of cases of retention, natural resolution occurs and the breeding potential is normal by 2-3 months after calving. Treatment: 1- Manual removal: The technique used for manual removal of RFM range from externally applied gentle traction, through to forced extraction and separation of each cotyledon and caruncle. The traditional method of manual removal has been described by Arthur and Bee. In this method, the post-cervical portions of the placenta were twisted together into a ‘rope’, then a hand was inserted into the uterus and each cotyledon was squeezed out of the base of the maternal caruncle. Continuous steady traction and rotational force were applied with the other hand to withdraw the detached membranes. The current recommendations for the manual removal of fetal membranes, there fore, are that cows should not be examined until 96 hours after calving and that removal should be gentle. 2- Ecbolic agents: Administration of oxytocin after calving (100 IU) to stimulate adequate uterine contractions. Oestrogenic substance have also been given, to increasing the sensitivity of the myometrium to oxytocin and enhancing the natural uterine defense mechanisms (I/M or I/uterine). Prostaglandin may assist in detachment of membranes through direct actions upon the placetomes. 3- No treatment: Arthur and Bee were convinced that uncomplicated cases of RFM left without treatment. 4- Treatment for metritis/ endometritis: Antibiotics can be given locally and systemically. 40 An-oestrus Syndrome: A failure of the cow to display oestrus. Causes of anoestrus include: a. Pregnancy. b. Ovarian inactivity, resulting anovulatory anoestrus. c. Failure to observe oestrus or ovulation is not accompanied by signs of oestrus (silent heat). d. Cystic ovarian disease. e. Miscellaneous conditions, such as spontaneous prolongation of the life span of the CL (infection). Anovulatory anoestrus: Is more common in horses and swine than in cattle and sheep. The animal shows normal behavioral oestrus and the ovarian follicle reach preovulatory size but do not rupture. Anovulatory follicles become partly luteinized and then regress during the oestrus cycle. Post-partum anovulatory anoestrus could almost be regarded as a normal aspect of bovine reproduction. Clinic al findings: o The clinical history is normally either of an animal that: a) Has not been seen in oestrus since the time of calving. b) Starting having oestrous cycles but has subsequently ceased. c) It is found to have relapse into an oestrus when presented for pregnancy diagnosis. o On examination per rectum, the ovaries of affected cows are small, quiescent and usually flat and smooth, especially in heifers. o In some anoestrus cows, follicles up to prematuration size of 1.5 cm may be present. Old cows frequently have roughened, irregular ovaries, because of the presence of old regressed corpora lutea and corpora albicantia. o Ultrasonographic examination of the ovaries per rectum permits identification of ovarian structures. o Assessment of milk or blood progesterone is helpful in confirming a diagnosis; two samples can be taken at 10 days intervals or a single sample 10 days before a rectal palpation is made. Predisposing factors: 1- Breed: A genetic effect has been implicated for the longer period for the return of ovarian function postpartum in beef breeds (36-70 days) compared with dairy breed. The predisposition of beef suckler cows to anoestrus may be due to inhibitory effect of prolactin. 2- Season: The effect of the season and environment is shown by increased frequency of anovulatory anoestrus. In late autumn or winter calving cows that have been in anoestrus, there is frequently a return to normal ovarian activity when there are turned out to grass in the spring (photoperiod). 3- Nutrition: Inadequate nutrition will increase the time interval to the first ovulation. Cows which are in negative energy balance during early lactation are more at risk of becoming anoestrus than those which are in energy equilibrium. Trace element deficiencies (magnesium, phosphor, copper) are associated with anoestrus. 41 4- Stress: Many sources of stress are believed to be affecting the cattle productivity, such as metabolic stresses, imposed by high yield and social stresses, produced by group and space management of animals, physical stressors, include transport, temperature and handling. Stresses of pain like lameness is predispose to an anoestrus, both due to its effect upon nutrition and directly via the stress produced by chronic or unrelieved pain. Lame cows have longer CCI and have low conception rates. Treatment: Anovulatory anoestrus can be treated in one of two main ways: 1- Elimination of predisposing factors: For example, feeding could be improved, micronutritient (minerals and vitamins) deficiencies corrected, stress reduced. 2- Hormonal treatment: Use of eCG (PMSG) to stimulate ovarian activity (3000-4500 I.U. This dose may also cause superovualtion). eCG is usually used with combination with P4. Administration of Gn-RH (5 mg single dose) to causes the release of LH. However in dairy cows in deep anoestrus, or in beef suckler cows, a second injection of Gn-RH 10 days later is necessary. In current practice, the main use of Gn-RH is in combination with PGF2α in so called 'GPG' regimens. GPG programmes are based on the injection of GnRH to cause luteinization of follicle, followed 7 days later by PGF2α to cause luteolysis, then 2days later with further GnRH, to cause ovulation of a new follicle. Alternatively, the response of GPG programms can be augmented by inserting P4 at the time of the first GnRH injection to improve pregnancy rate (prevent premature luteolysis after administration of PGF2α and makes cows more likely ovulate). Progesterone treatment (PRID) (CIDR), often associated with other drugs such as oestrogens (oestradiol has been given at the time of withdrawal P 4. Recently it will administer at the time of placing P4 insert, in order to restart the follicular waves and given as ester; benzoate or cypionate), Gn-RH or PGF2α has long been used to induce ovarian activity post-partum. Oestrogens, both natural and synthetic, have also been used to treat anoestrus. Cystic ovarian disease (COD): Ovaries are said to be cystic when they contain one or more fluid-filled structures (cyst) larger than a mature follicle (> 2.5 cm diameter), which are persistent for longer than 10 days which result in aberrant reproductive function. In the normal postpartum cow, follicular waves start soon after calving, with ovulation of the dominant follicle of one of early waves. COD is clinical manifestation of dysfunction of this process, arising as a result of anovulation of a dominant follicle. More ovarian cysts were detected in cows examined at 30 days post-partum. Ovarian cysts are either follicular or luteal (nymphomaniacal or acyclic). Predisposing factors: Hereditary predisposition. Incidence of cystic ovaries is influenced by the cow’s sire and is more common in Holestein Friesian than the other dairy breeds. Beef breeds are seldom affected. The incidence of disease is greatest in animals between 4 and 6 years of age and in high-yielding cows. It is uncommon in animals in their first lactation. The feeding of high-protein diets appears to be a contributory factor. 42 Season of the year. The disease is more prevalence in winter than at other times of year, because cow have reached peak yield, lack of exercise, excess dietary protein and/ or effect of photoperiod. Ketosis, dystocia, twin births, RFM and milk fever are considered risk factors for condition. Aetiology: 1. Few cases of cystic ovarian disease are caused by mechanical interference with the process of ovulation (ovarobursal adhesion). 2. Failure of the endocrine mechanism of ovulation (more common cause). Cysts occur when insufficient LH is released to cause normal ovulation. Conversely, high concentrations of LH during follicular phase may associate with the development of cysts. 3. Stress-induced elevatations of ACTH concentrations that cause abnormal LH surge. 4. The prolactin-thyroid system may be involved in the development of ovarian cysts. Hypothyrodism has been associated with cystic follicles, in a number of species, and thyroxine concentrations are negatively correlated with milk production (high yielding cows have lower concentrations). 5. Defects within the ovary. The ability of follicles to respond to pre-ovulatory LH surge is dependent upon the timely induction of LH receptors during follicular maturation; if too few receptors are available then ovulatory failure may occur. Clinical signs: The main clinical signs of cystic ovarian disease in cattle are nymphomania, anoestrus or masculinization. Cow with follicular cysts are often nymphomaniacal i.e. displaying excessive, prolonged signs of oestrus and a shortened interval between successive heats (irregular). There is odematous swelling of the vulva, frequent and copious discharge of clear mucus, relaxation (sinking) of the sacro-sciatic ligaments. Affected cows may have nervous disposition, with depressed milk yield and loss of bodily condition. The luteal or luteinized cysts result in a cessation of cyclical activity. If cows with luteinised cysts are left untreated then a proportion of they will become virilised. These individuals will develop a masculine conformation (mount to other cows). Diagnosis: a. Traditionally, ovarian cysts have been classified as either follicular or luteal cysts. Follicular cysts are thin-walled and have little or no luteal tissue in the cyst wall (single or commonly multiple cysts). Luteal or lutinized cysts are thick walled and more usually single and have a large quantity of luteal tissue in the cyst wall. Cysts can be confused (per rectum) by corpus haemorrhgicum, pre-ovulatory follicle, abscesses and tumours. b. Measurement of progesterone concentration in blood plasma/ serum (> I ng/ml) or milk (> 2 ng/ml) is used to determine type of cyst (high concentration in luteal cysts). c. The accuracy of diagnosis can be improved by the use of trans-rectal ultrasound imaging. The thicker wall (> 3 mm) of the luteal cysts allows differentiation from follicular cysts. Treatment: I. Spontaneous recovery from COD occurs frequently in the early post-calving period (self cure rate 15- 30 % of cases within 45 days after calving). 43 II. The earliest method of treating cysts was by manual rupture per rectum. But this method may cause trauma or haemorrhage and result in ovarobursal adhesions. III. Hormonal treatment: o The choice of hormonal treatment depends upon the type of cyst that is present. o Follicular cysts treated by hCG (3000-4500 IU I/V) or GnRH that cause release of endogenous LH. Thus the GnRH includes luteinisation of the cyst or formation of new corpora lutea (increase progesterone concentration). Doses of 100-250 µg of GnRH causes luteinisation while use of GnRH analogues (buserelin 10 µg) or large doses of GnRH 0.5-1mg has been associated with ovulation of follicle and formation of new CLs. o Follicular cyst may also be treated with P4; signs of nymphomania disappear within 24 hours of PRID insertion, the cysts gradually regress and, following removal after 10-12 days, there is oestrus with ovulation and CL formation. o Luteal cysts can be treated effectively by PGF2α (may be treated by GnRH, hCG or progesterone). Combination of GnRH and PGF2α (9 days later) may reduce the interval between treatment and first service and increase progesterone concentration. Consequences: Cystic ovarian disease depresses fertility in number of ways; it extends the calving interval, decreases life time milk yields and increases the involuntary culling rate. Consequence of cystic ovarian disease is the development of mucometra. The Repeat Breeder Syndrome:Repeat breeder females return to service repeatedly after being bred to a fertile male. A repeat-breeder cow exhibits normal signs of oestrous every 18 to 24 days but requires more than three services to become pregnant. The incidence of repeatbreeding is higher in dairy herds using artificial insemination rather than natural service. Both fertilization failure and embryonic deaths at much higher rate than in normal cows. Errors in oestrous detection may also contribute to repeat returns to service in dairy cows. Three main causes of repeat breeding remain as causes of an impaired uterine environment: abnormalities of the peri-ovulatory period, chronic degeneration of endometrium and luteal deficiency. (1) periovulatory abnormalities: A number of studies of repeat breeder cows have shown endocrine abnormalities of periovulatory period. Repeat breeders are likely to have delayed ovulation and extended follicular phase. There is a longer interval between luteolysis and ovulation and delayed LH surge, resulting in follicles and oocytes are relatively aged by the time of ovulation. As a result of the impairment of the events around ovulation, repeat breeders are also more likely to have a delayed post-ovulatory rise in P4 concentrations. (2) Damage to the endometrium: Mild chronic endometritis is one of the most common causes of repeat breeding. Uterine secretions of repeat breeder cows have, generally been characterized as differing from those of normal cows. (3) Luteal deficiency: Insufficient progesterone due to inadequate function of CL (main source of progesterone) is lead to pregnancy failure. 44 Diagnosis of luteal deficiency by rectal palpation it is impossible and by taken single sample of blood or milk progesterone for analysis is also of little value. Treatment:1) Use of GnRH or hCG 11- 13 days after breeding to induce the accessory corpora lutea or augment the activity of CL. GnRH may be used at the time of inemination to improve pregnancy rates. 2) Progesterone implants have been used to try to augment progesterone concentrations during the period when luteolysis is expected. Abortion:It is defined as termination of pregnancy with expulsion or production of fetus (fetuses) between 152 and 270 days of gestation in cows. The fetus may born dead or survives for less than 24 hrs. Abortions may be spontaneous or induced, infectious or non infectious. Spontaneous abortion is more prevalent in cattle (dairy cattle) and may also occur in animals bred immediately after puberty or after parturition. Non infectious abortion may be due to chemicals (Nitrates), drugs (Oxtra), Hormonal (oestrogens, PGF2α), malnutrition, genetic or chromosomal, (anomalies) or physical (fever, stress). Infectious causes of abortion include:1. Brucella abortus; occurs at 6 – 9 months. 2. Leptospira spp; occurs at 6 – 9 months. 3. Listeria monocytogenes; occurs at 6 – 9 months. 4. Compylobacter fetus (5 – 7 months). 5. Trichomonas fetus; occurs before 5 months. 6. Neospora canium. (3-8 months by mean 5.5 months). 7. Salmonella spp. (dublin); its usually sporadic. 8. Arcanobacteium pyogens; is usually sporadic and occurs at any stage. 9. Mycobacterium tuberculosis; occur at any stage. 10. Aspergillus spp; occurs from 4 months to term. 11. Bacillus licheniformis (sporadic, late abortion). 12. Infectious Bovine Rhinotracheitis (IBR); occurs at 4 – 7 months of gestation. 13. Bovine viral diarrhoea (BVD) virus; occurs at any stage. 45 Infertility in the mare: Infertility can be divided into: 1) Mares that fail to cycle. 2) Mares that cycle normally but don't conceive. 3) Mares that cycle normally and conceive but then suffer from early embryonic death (EED). Broadly, they can be categorized into infectious or non-infectious factors, with the latter being further divided into structural abnormalities and functional aberrations. Structural abnormalities of the female reproductive tract: Vulva: In the normal mare, the vulva provides the first effective barrier to protect the uterus from ascending infection. The ‘normal’ mare has three functional genital seals forming a barrier between the external environment and the uterine lumen: the vulva, the vulvo-vaginal constriction and the cervix. During oestrus, the vulva and cervix relax, leaving the vulvo-vaginal constriction as the only seal. The vulval lips should be full and firm and meet evenly in the midline with 80% or more of the vulval opening below the brim of the pelvis. If the vulval seal is high in relation to the pelvic brim, the vestibular seal is incompetent and there will be aspiration of air with bacteria and contaminated material into the vagina (pneumovagina; also called ‘wind-sucking’). The initial vaginitis may lead to cervicitis and acute endometritis resulting in subfertility. Furthermore, the pneumovagina may lead to urovagina (urine pooling within the vagina) when the vestibule and urethral opening are displaced cranially. The more severe conformational abnormalities are more likely to result in failure of the vulval seal, and to increased faecal contamination since the vulva forms a shelf on to which faeces may collect. Older multiparous mares are more commonly affected with pneumovagina. In some mares, pneumovagina may only occur during oestrus when the perineal tissues are more relaxed. Treatment should be directed at correcting the cause of pneumovagina, and concurrently treating the resulting acute endometritis. The former can be done surgically by Caslick’s operation. The mare should be suitably restrained and her vulva thoroughly cleaned and dried. With a gloved hand, the level of the floor of the pelvis is determined. This allows you to ascertain the level to which the dorsal commissure of the vulva must be sutured. Beginning at this level, the mucocutaneous junction of the vulva is infiltrated with local anaesthetic through a 21-gauge 1-inch needle. Using rat-toothed forceps and scissors a very thin (no more than 4 mm) strip of mucosa should be removed from the anaesthetised area. The exposed submucosal tissues are sutured together using simple interrupted sutures or a locking pattern. Antibiotics are not given, but tetanus prophylaxis is needed if the mare is not vaccinated. The aim of the operation is to reduce the vulval aperture and so prevent pneumovagina and faecal contamination of the vestibule. 46 The time of suture removal is not crucial, and is normally done approximately 2 weeks after surgery. However, the vulva must be re-opened by performing an episiotomy before the next foaling, otherwise major injury can result. Mares that require natural mating subsequently may also need to have an episiotomy if the size of the vulva has been greatly reduced. The episiotomy wound should be repaired soon after foaling or mating to prevent pneumovagina. If there has been severe trauma to the vulva at foaling, it maybe necessary to wait for the tissue swelling to subside before attempting repair. Vulvo-vaginal constriction: A complete persistent hymen can also occur, which can result in the accumulation of fluid within the vagina and uterus due to impaired natural drainage. Sometimes the hymen may be so tough that it can only be ruptured using a guarded scalpel blade or scissors. The small incision can then be enlarged using the fingers and hand. Vesico-vaginal reflux, also known as urovagina or urine pooling, is the retention of incompletely voided urine in the cranial vagina due to an exaggerated downward cranial slope of the reproductive tract. Clinical signs can include urine dripping from the vulva, urine scalding and a history of failure to conceive. Diagnosis is easiest using a speculum examination during oestrus to detect urine in the cranial vagina. Uterine infection with an accumulation of exudate in the vagina can be confused with the condition. Cervix: A distinctive feature of the equine cervix is its dilatability, and the absence of rigid, annular constricting rings seen in farm animals. The cervix fails to relax properly during oestrus, so that fluid is unable to drain and accumulates in the uterine lumen. Failure of the cervix to open during oestrus can lead to unwillingness of the stallion to complete mating or ejaculate intra-vaginally. Failure of the cervix to close during dioestrus can lead to persistent endometritis and failure to conceive, or early embryonic death. Failure to maintain closure during pregnancy can lead to gestational failure. Assessment of the cervix must form a part of the routine pre-breeding examination of a mare, either directly using a speculum per vagina and/or by digital exploration, preferably during dioestrus when it is more tightly closed under the influence of progesterone. Injury, resulting in cervical incompetence or fibrosis, most often occurs during parturition. This is especially the case if fetotomy is performed by an inexperienced clinician, or without adequate instrumentation. The cervix can also be damaged by irritant chemicals such as povidone-iodine. Developmental abnormalities of the cervix have been described; these include aplasia and a double cervix. Uterus: Uterine cysts are the most common type of uterine lesion identified in the mare. Two distinct morphological types are recognized: (1) endometrial cysts, which are usually 2 cm or less in diameter, and (2) lymphatic cysts, which are generally larger. Although they can be diagnosed at post-mortem examination, the use of ultrasonography has shown that the incidence is much greater than was originally suspected. 47 The relationship between sub-fertility and uterine cysts is not clear. Some authors suggest that uterine cysts can reduce pregnancy rates. The effect on fertility could be by restricting early conceptus mobility, whilst later in pregnancy they may interfere with nutrient absorption because of contact between the cyst wall and yolk sac or allantois. The Cysts can be confused with an early conceptus and give rise to false positive early pregnancy diagnosis or the incorrect diagnosis of twin pregnancies during ultrasound scanning. Differentiation is based on previous cyst mapping, but also the early mobility of the conceptus, the presence of specular reflections, the conceptus’s spherical appearance and growth rate. The need for treating endometrial cysts is uncertain. If a mare is found at the beginning of the breeding season with a large number of cysts, it is generally best to continue to attempt to get the mare in foal that season. If she fails to become pregnant, some form of treatment should be attempted and an endometrial biopsy should be taken to help determine the likelihood of her carrying a foal to term. Larger cysts can be punctured using an endometrial biopsy instrument or manually if the cervix allows passage of one hand. Most uterine cysts involve the endometrium, but occasionally an extra luminal uterine cyst lying external to the endometrium can be identified on ultrasound examination. Uterine adhesions are most frequently diagnosed on endoscopic examinations of the uterus. Multiple adhesions adversely affect fertility by causing fluid accumulation or by affecting the mobility of the conceptus. Severe adhesions can completely obstruct one or both of the uterine horns. It is possible to remove the obstruction endoscopically by either cautery or laser techniques, starting at the thin membranous parts of the obstruction. After removing the obstruction, the uterus should be flushed to remove any debris and the mare treated with PGF2α. Ovarian neoplasia: Ovarian neoplasia is uncommon in the mare although many types of tumour have been described, with the granulosa theca cell tumors (GTCTs). GTCTs arise from the sex cord stromal tissue within the ovary and may be hormonally active, producing variable amounts of steroids that cause behavioural changes and alteration to normal cyclical activity. Mares can exhibit nymphomania, anoestrus or aggressiveness with signs of virilism(clitoral enlargement, stallion-like conformation). The secretion of high amounts of inhibin by the neoplastic granulosa cells inhibits follicle stimulating hormone (FSH) secretion, and is thought to be the reason for atrophy of the contralateral ovary. A GTCT often appears ultrasonographically as a large (7–40 cm) spherical mass with a multi cystic or ‘honeycomb’ appearance. Unilateral ovariectomy is the only satisfactory treatment for GTCTs, since the prospect of breeding from the mare is extremely poor unless the neoplastic ovary is removed. Functionally Infertility: Mares are seasonally polyoestrous, and environmental and other factors can exert a profound effect on reproductive function, particularly during the transitional period between winter anoestrus and the onset of cyclical activity in the spring. 48 Anoestrus due to ovarian acyclicity: Winter anoestrus: the onset of cyclical activity is stimulated by increased day length. During winter months mares are normally acyclical. On rectal palpation or transrectal ultrasound imaging both ovaries will be small, and in some mares there will be a number of small follicles. Plasma progesterone concentrations are >1 ng/ml. Prolonged anoestrus can be prevented by good management. Progesterone withdrawal therapy (PRID) has been used successfully. Lactation-related anoestrus: is commonest in mares foaling early in the season. Affected mares may have a normal postpartum oestrus after 6–12 days, but fail to return to oestrus at the end of the first dioestrus. Alternatively they may not even have a normal ‘foal heat’.The ovaries resemble those of a mare in deep winter anoestrus( small and inactive); the condition can last for several months. Affected mares should be teased and examined weekly per rectum to assess their ovarian status. Treatments similar to those described above for winter anoestrus have been used, but with little success. Anoestrus caused by a prolonged luteal phase: Persistence of luteal activity: is a major cause of subfertility in the non-pregnant mare. Traditionally, the term ‘prolonged dioestrus’ has been used to describe a condition where the corpus luteum persists beyond its normal cyclical life span of 15/16 days, resulting in the maintenance of elevated circulating progesterone concentrations for longer than expected. Plasma progesterone profiles are indistinguishable from those of pregnant animals. The uterus becomes firm and tubular (tonic) and the cervix is typical of that of pregnancy. Transrectal ultrasound imaging fails to detect a conceptus. Treatment is by the injection of a luteolytic dose of PGF2α or a synthetic analogue. Pseudo pregnancy: pseudo pregnancy is a term used to describe a syndrome in which non-pregnant mares that have been mated do not return to oestrus. It occurs if there is early embryonic death after 15 days of gestation with persistence of the corpus luteum resulting in a prolonged luteal phase. The cervix remains tightly closed, and the uterus is tense and tubular. It is differentiated from pregnancy by the absence of a conceptus on ultrasound examination. Silent oestrus: Some mares either do not show oestrus, or are slow to show detectable signs using standard teasing methods despite the fact that ovulation occurs; this is called silent oestrus. The degree of reduced expression of oestrus varies from partial (sub-oestrus) to complete (anoestrus). Rectal and vaginal examinations confirm that the mare is in oestrus and has follicles of an ovulatory size. It is essential to distinguish the condition from a prolonged luteal phase in which there is also follicular development. The treatment is based on thorough and careful teasing. Frequent and persistent teasing may persuade the mare to show oestrus. To breed mares naturally during a silent oestrus, some form of restraint may be necessary; many mares approaching ovulation accept the stallion when twitched and hobbled. An intramuscular injection of oestradiol benzoate (10–20 mg) 6 hours before breeding can be tried as a last resort. 49 Transitional ‘spring’ oestrus: Because of the considerable variation in the duration of oestrus during the transitional period, efficient breeding of the mare can be difficult. Shortly after the winter solstice, changes in the pineal/hypothalamic/ pituitary axes result in some follicular growth; however, follicles remain small, do not ovulate, and regress. Eventually, after a variable transitional period of up to 2 months, larger follicles (>35 mm) will develop and ovulate, usually heralding the onset of normal cyclical ovarian activity. During the transitional period the behavior is variable, ranging from total rejection of the stallion, to interest but resistance to him mounting, to normal acceptance. The diagnosis is by thorough ultrasonic examination and rectal palpation, which reveals transitional follicles reaching a preovulatory size of > 30 mm. Visual identification of a corpus luteum or progesterone levels above 4 ng/ml confirm that the first ovulation has occurred and hence the onset of normal ovarian cyclical activity. The treatment of mares in the transitional stage is based on progesterone with or without the addition of oestradiol. Progesterone can be administered as an oilbased intramuscular injection, orally as the synthetic progestogen altrenogest (Equine Regumate) or by using a silastic progesterone releasing intravaginal device. There has been much interest recently in using GnRH or its analogues, administered by injection, infusion or subcutaneous implant, to hasten ovulation in transitional or even anoestrous mares. Regardless of the hormones used, mares undergoing treatment early in the season need 16 hours of adequate light and good housing and nutrition to ensure success. Chromosomal abnormalities: The normal chromosome complement for the domestic horse is 2n = 64. The incidence of chromosomal abnormalities is difficult to assess, but must be suspected in maiden mares with small, inactive ovaries and an immature tubular genital tract. The main karyotypic abnormality of such mares is the gonadal dysgenesis (Turner’s syndrome .63, XO). These mares are usually small for their age and do not cycle, although occasionally they may show passive oestrous signs. On palpation per rectum, their ovaries are very small, smooth and firm. The uterus is small and flaccid (a thin band). These mares are sterile. Other chromosome abnormalities include ovarian hypoplasia and testicular feminisation.(pseudohermaphrodite mares ). Embryonic death: In normal fertile mares the fertilisation rate is more than 90%, which is comparable with other domestic species, with estimates of the EED rate at between 5 and 24%.The differences in the estimates are due to varying methods of pregnancy detection, and the animals studied. There are many factors caused EED include: a) External factors such as stress, nutrition, season of the year, climate, sire effects and trans- rectal palpation. 50 b) Abnormal maternal factors including hormone deficiencies and imbalances, uterine environment, age and lactation. c) Embryonic abnormalities are also important to consider in relation to embryonic death. Ultrasonic scanning has provided a valuable tool in studying embryonic death. There are certain morphological features detected with ultrasound that are typical of mares in which embryonic death is occurring. Some of the consistent features include: (1) presence of fluid within the uterine lumen; (2) prominent endometrial oedema; (3) decreased or prolonged conceptus mobility; (4) undersized or irregularly shaped conceptus; (5) cessation of embryonic heart beat; (6) reduced volume of placental fluids; (7) disorganisation of placental membranes. Fetal death and abortion: Abortion is defined as expulsion of the fetus and its membranes before 300 days, whereas a stillbirth is expulsion of the fetus and its membranes from day 300 onward. The causes of equine abortion can be broadly divided into non-infectious (70%), infectious (15%) and unknown (15%). When abortion occurs, the mare should be isolated, a history obtained and the fetus sent to an approved laboratory for necropsy. Non-infectious causes of abortion and stillbirth: a. Twinning. b. Umbilical cord abnormalities. The normal length of umbilical cord ranges from 36 to 83 cm (mean 55 cm). Increased cord length (over 80 cm total length) has been associated with excessive cord torsion, which can cause twisting of the umbilical blood vessels (lead to fetal death and abortion). Decreased cord length can cause premature tearing of fetal membranes, leading to fetal asphyxia. c. Premature placental separation. Causes of premature placental separation are largely unknown. When placental separation occurs shortly before parturition, the thickened placenta often does not rupture through the cervical star, and the allantochorion bulges out of the vulva (‘red bag’ delivery). d. Body pregnancy. In this condition almost the entire chorionic surface of the placenta contained within the uterine body is without villi, while that contained within the horns is covered with an excessive number of villi. The proportion of the placenta corresponding to the two uterine horns is small, and the fetus is situated entirely within the uterine body. The abortion occurs when the nutritional demands of the fetus exceed the ability of the placenta to meet them. e. Fetal abnormalities. f. Maternal disease. Pyrexia and malnutrition during pregnancy have been implicated as causes of abortion. Infectious causes of abortion: The main causal agents of infectious abortion are viruses, bacteria and fungi, more rarely mycoplamsa and protozoa. Viral abortion. Viruses that can cause abortion in mares include: equine herpes virus EHV, equine viral arteritis and equine infectious anaemia. EHV is the single most important infectious cause of equine abortion. The disease is caused by EHV-1 and, rarely, EHV-4. The source of the virus is: Clinically affected animals with nasal secretions. Aborted fetuses and their membranes. Infected foals born live at term. The majority of EHV-1 abortions occur in the last 4 months of gestation. The mare shows no signs of impending abortion or clinical disease. 51 Equine viral arteritis(EVA) is a contagious viral disease of the horse. The two important routes of EVA transmission are venereal from a stallion with infected semen, and aerosol via the respiratory secretions of an acutely infected horse. Naturally occurring abortion from EVA is not common. It occurs sporadically, sometimes in clusters. Abortion occurs as a result of myometrial necrosis and oedema leading to placental detachment, and hence fetal death. Abortions tend to occur in the latter half of pregnancy. Acute EVA can be confirmed by virus isolation from nasopharyngeal swabs, heparinised blood samples, and urine and semen samples. Diagnosis of abortion due to EVA is largely dependent on virus isolation from the placenta or fetal tissues; there are no pathognomonic gross lesions. Bacterial abortion. There are 3 proposed routes of infection: A. The first involves the hypothesis that bacterial levels in the uterus can be low enough to allow conception, but multiply during gestation producing placentitis and abortion. B. The second involves hematogenous spread of a pathogen. C. The third and most common route involves transcervical migration of the pathogen. The placentitis often leads to placental insufficiency with abortion of a growthretarded fetus. The placenta is often thickened and covered with exudate and the fetus septicaemic. They are often opportunist pathogens that can be isolated from the caudal genital tract of normal mares, i.e. Streptococcus spp. and Escherichia coli. Others are considered to be venereal pathogens, i.e. Pseudomonas spp. and Klebsiella spp (recently Leptospira pomona and Salmonella abortus equi) . Fungal abortion. Aspergillus spp. are the most common cause of mycotic placentitis and mycotic abortion in the mare. Fungal disease has a similar pathogenesis to bacterial abortion with inflammation of the chorion beginning at the cervical pole. Endometritis: Endometritis can be categorized as: venereal infection (sexually transmitted diseases ). chronic infectious endometritis. endometrosis or pyometra (chronic degenerative endometritis). persistent mating-induced endometritis (delay in uterine clearance). venereal infection: When we speak of sexually transmitted endometritis, generally we are referring to Contagious Equine Metritis (CEM). This disease is due to Taylorella equigenitalis .It can survive for an extended period on the external genitalia of the stallion and the vagina or clitoris of the mare. When infected, the stallion may show no clinical signs. The mare shows acute endometritis with a thick, grey, mucoid discharge after breeding and may short cycle due to the inflammation. The acute signs subside rapidly with some mares remaining as asymptomatic carriers. Diagnosis. Before the breeding season, swabs should be taken from the clitoral fossa, clitoral sinuses and the vestibule.In addition, in stallions, two sets of swabs must be taken from the pre-ejaculatory fluid (if possible), penile sheath, urethra and urethral fossa. A diagnosis of endometritis can be made by collection of 52 concurrent endometrial swab and smear samples during early oestrus for bacteriological culture and cytological examination, respectively. Treatment: I. Topical treatment of the stallion's penis with chlorhexidine scrub and nitrofurazone seems to be effective. II. Topical treatment of the mare consists of IU antibiotics and flushing the clitoral fossa and sinuses with chlorhexidine then packing them with chlorhexidine or nitrofurazone. III. Surgical ablation of the clitoral fossa & sinuses is used to treat the carrier mare and is required for export/import by some countries. Certain isolates of Pseudomonas and Klebsiella may be transmitted venereally. However, Pseudomonas and Klebsiella may also be isolated from the external genitalia of normal stallions. chronic infectious endometritis: Chronic endometritis results when the barriers to contamination of the uterus are compromised or when the uterus that is susceptible to persistent mating induced endometritis is subjected to repeated insults (i.e. breedings). The anatomic barriers to contamination of the uterus are the vulva, vaginalvestibular sphincter and cervix. Poor perineal conformation may contribute to the establishment of chronic endometritis. Etiologic agents most commonly found in cases of chronic endometritis are Strep. equi zooepidemicus (the most common), E. coli, Pseudomonas aeruginosa, Klebsiella pneumonia and yeast. The first step in treatment should be to correct any anatomical defects. Antibiotics are often used to treat a bacterial infection before breeding or as routine post breeding treatments. Endometrosis: Endometrosis can be defined as the collective term to describe the wide range of degenerative changes (fibrosis and glandular degenerative changes). There are several possible etiologies for pyometra in the mare. Cervical adhesions or malfunction with failure to drain contents are often implicated. There is usually no sign of systemic illness. In some cases there may be a discharge. On rectal exam, a fluid filled uterus is found which must be differentiated from pregnancy. The majority of affected mares cycle normally. In some cases, with severe endometrial damage, PGF release may not occur so that luteal function is prolonged. In other cases, the mare may cycle at shortened intervals due to endometritis and PGF release. Successful treatment of endometrosis is difficult .The treatment has involved the use of mechanical and chemical agents and that cause endometrial necrosis and the prognosis for future fertility is poor. Persistent mating-induced endometritis: At coitus, the mare’s uterine lumen becomes contaminated with microorganisms and debris. Even if mares are bred by artificial insemination, semen is deposited directly into the uterus. In addition, it has recently been shown that spermatozoa without bacterial contamination induce a uterine inflammatory response. All mares have a transient post breeding endometritis which is considered physiologically normal and most mares are able to clear the endometritis in a timely fashion. 53 Endometritis becomes established or persists when the natural defense mechanisms fail. Much effort has gone towards determining why some mares develop a persistent endometritis after breeding while others are resistant. a) It appears now that the primary defect is a failure of mechanical clearance. b) Susceptible mares have reduced clearance due to a number of factors including reduced lymphatic function, reduced myometrial response, conformation, etc. c) This is probably a gradual process resulting in a loss of efficiency in bacterial clearance, although it can occur abruptly following serious trauma. Probably one of the best ways to diagnose persistent post mating induced endometritis is to observe an accumulation of fluid by ultrasound postinsemination. If on examination at 12 -24 h post mating fluid is observed in the uterus, a diagnosis of persistent post mating endometritis can be made. The aim of treatment should be to assist the uterus to expel the normal inflammatory products arising from the response to breeding. The successful management of susceptible mares should logically require some form of post-mating therapy such as intrauterine antibiotic infusion, uterine lavage and intravenous oxytocin; these may be used alone or in combination. Management protocol useful in the highly susceptible mare: A. Overall management of such mares must be excellent prior to breeding. B. Good hygiene at foaling is essential and all mares should be thoroughly examined postpartum for the presence of trauma. C. Gynaecological examinations, particularly of the vagina, should be performed as aseptically as possible. D. Thorough digital examination of the cervix can identify fibrosis,lacerations or adhesions that may need treatment before breeding. E. Attention should be paid to hygiene at mating by using a tail bandage and washing the mare’s vulva and perineal area with clean water. F. Breeding should occur at the optimal time, and the number of breedings should be minimized. This means that these mares need very close monitoring of the oestrous period by rectal palpation and ultrasonography. Dourine: Trypanosoma equiperdum causes a venereal disease called dourine, which is currently prevalent in Africa, the Middle East and Central and South America; it has been eradicated from Europe and North America. It affects horses, mules and donkeys of either sex. The initial sign is a non-painful swelling of the external genitalia of both stallions and mares; mares show a vaginal discharge and stallions have a paraphimosis. Some weeks later, depigmented areas and urticaria-like raised plaques 2–10 cm in diameter appear over the body surface. The disease is characterized by a low morbidity, but a high mortality of 50–75%. Diagnosis of dourine is made from the clinical signs, particularly the skin plaques, together with demonstration of the trypanosome in the discharges and in the skin lesions. Treatment using quinapyramine sulfate has been attempted, but stallions that recover may become carriers. 54