PowerPoint
advertisement

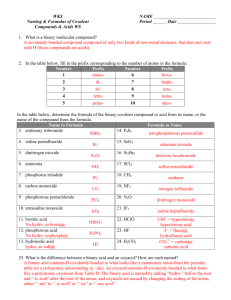
NAMING COMPOUNDS Chemical Formula A chemical formula is used to show the composition of the compound. Ex 1: H2O – this formula shows that there are 2 hydrogen atoms and 1 oxygen atom in a single water molecule. Ex 2: Al(NO2)3 – this formula shows that there is 1 aluminum atom, 3 nitrogen atoms and 6 oxygen atoms. Notice how you multiply the subscripts together to get the total number of atoms involved for oxygen. Binary Ionic Compounds A binary ionic compound is a compound consisting of 2 different elements, 1 metal and 1 non metal. For Example: NaCl Notice how the metal is always written first, then the non metal Naming Binary Ionic Compounds When naming a binary ionic compound, you always want to name the metal first, then the non-metal ion, changing the non-metal’s ending to –ide. Lets look at our last example again: NaCl Na would be ‘sodium’ and Cl is ‘Chlorine’, but we need to change the ending to –ide. So, the name to this compound would be ‘Sodium Chlor-ide’. Example 2 for Binary Ionic Compounds: Example 3 for Binary Ionic Compounds: Here is a list of the most common polyatomic ions: (It is a good idea to keep this list on hand ! You can find it as a link within the lesson) Naming Polyatomic ions: When naming polyatomic ions, they have specific names that have already been given to them, so there is no need to change their ending. Lets start with a simple example: NaOH If you refer back to the list, you will see that ‘OH’ is ‘hydroxide’. So, the name of this compound would be ‘Sodium Hydroxide’. Example 2: Naming Polyatomic Ions (NH4)2S To name this compound, it starts with a polyatomic ion, so you will need to go to your list to find the name. You will find that (NH4) is ammonium. Then, the non-metal is not a polyatomic ion, so we will have to change the ending to –ide. So, the final name of this compound is ‘Ammonium Sulfide’. Lets Reverse the Process!! Since you can now NAME the compounds, lets try to give the compound based on the name! For Example: Calcium Sulfate We know that ‘Calcium’ is represented by Ca. We will need to look back at our chart to see what ‘sulfate’ is….SO4^-2 Since Ca has a charge of +2 and SO4 has a charge of -2, our final product will be: CaSO4 Reverse It One More Time! Example 2: Aluminum Nitrate We know that aluminum is ‘Al’, but again, we must look at our chart for ‘Nitrate’. Nitrate is represented as – NO3 with a -1 charge. Since Aluminum has a charge of +3, the formula will be: Al(NO3)3 What If The Element Has More Than 1 Possible Charge! Yikes!! We know all the charges for the elements in groups 1-2 and groups 13-17, but what about those transition metals? Lets look at an example of how this could happen… Example 2: Switchin’ It Up! CrF3 Since Cr is a transition metal, we do not know what charge it has, so what do we need to do to find this out. We must work backwards. We know the charge of Fluorine is -1 since it is in group 17. It took 3 ‘-1’ charges to bond with Cr. So, we must find out what the charge of Chromium is to make the overall charge zero. (1 x ?) + (3 x -1) = 0 This means that each Chromium ion has a charge of +3. So, the name of this compound would be Chromium (III) Fluoride Example 2: PbSO4 Pb- lead- is our transition metal and SO4 is our polyatomic ion. Lets refer back to our polyatomic ion sheet to see what the name and charge of SO4 is…. Sulfate with a charge of -2 It too 1 (-2) sulfate atom to neutralize a single lead atom. (1 x ?) + (1 x -2) = 0 Lead has a charge then of (+2), so this compound is Lead (II) Sulfate Naming Binary Covalent Compounds: Here Is The List Of Prefixes: (Again, this is another list to keep on hand ) Lets Try A Few!! Ex 1: N2O – Dinitrogen Monoxide Ex 2: SO3 – Sulfur Trioxide Now, you try the rest…. Ex 3: NO – Ex 4: CF4 – Ex 5: P2O5 – Ex 6: N2O3 - OXYACIDS These are compounds that include hydrogen, oxygen and a third element. Many of the polyatomic atoms from our chart are produced by the loss of hydrogen ions from oxyacids. That is why the name of the polyatomic ion is used in the name of the oxyacid as well. If the poly atomic ions name ends in ‘-ate’, then change the ending to ‘-ic’ and name the oxyacid. Do not put ‘hydro’ at the beginning of the name for any oxyacid, as this is only done in binary acids, which we will look at next. If the polyatomic name ends in ‘-ite’, change it to ‘-ous’. Examples: (‘-ate’ to ‘-ic’) Example 1: HNO3 – Nitric Acid Example 2: H3PO4 – Phosphoric Acid Example 3: H2CO3 – Carbonic Acid Examples: (‘-ite’ to ‘-ous’) Example 1: HNO2 – Nitrous Acid Example 2: H2SO3 – Sulfurous Acid (Almost Finished!) Naming Binary Acids Binary acids consist of hydrogen and a non metal, other than oxygen. Here is the general format to name a binary acid: Lets Practice: HF – Hydrofluoric Acid HI – Hydroiodic Acid Now, you try some…. HCl – HBr – H2S –