Chapter 7
advertisement

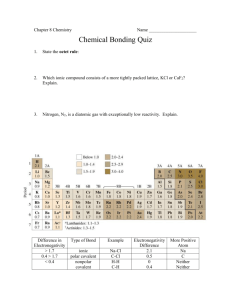
Naming Ionic Compounds and Covalent Molecules •Ionic compounds do not use prefixes in naming. This is because the ions only bond in a ratio of their charges • Covalent molecules require the use of prefixes in naming molecules. Writing chemical formulas Use the elements symbols from the periodic table H2O H is symbol for Hydrogen O is symbol for Oxygen The # 2 is written to show the number of H, this is call a subscript Metals have many charges Roman Numerals- used in ionic compounds for metals Gold (I) ion is Au+ Using symbols to represent numbers Gold (II) ion is Au2+ Iron (II) ion is Fe2+ Iron (III) ion is Fe3+ Charges Cu+ or Cl- the + and – represent the charge Cu 2+ is the charge on this particular atom of Copper. This is called superscript Naming Cations Use the names of the elements. K+ know as potassium ion Zn2+ know as zinc ion When an element forms two or more ions, the ion names include roman numerals to show charge ◦ Cu+ = copper (I) ion ◦ Cu2+ = copper (II) ion Naming Anions Name is formed from the element, but ends in –ide ◦ Cl- is the chloride ion ◦ O2- is the oxide ion Binary Ionic Compounds Binary means compound is made up of just two elements. Examples: NaCl,CaF2, and AlCl3. Named as Sodium Chloride, Calcium Fluoride and Aluminum Chloride. (Notice they do not have prefixes in the names.) Cation followed by the name of the anion ◦ Positive then negative Compounds must have no overall charge Polyatomic Ions Charged groups of two or more bonded atoms that can be considered a single ion Example is Phosphate (PO4)-3. Charge is either positive or negative and the number after the sign. Many contain oxygen ◦ -ite, and –ate indicate the presence of oxygen ◦ examples: Sulfite and Sulfate Polyatomic Ion form IONIC bonds Polyatomic ions are negative ions, except for ammonia, and they are found at the end of the chemical formula. Example is Sodium Phosphate They bond in a ratio of their charges. Na+1 and (PO4)-3 are their charges Na3(PO4) is the compound’s chemical formula. Covalent Molecules A bond formed when one or more PAIRS of electrons are shared between atoms. Requires the use of PREFIXES, since covalent bonds may occur in many ratios of atoms, so we must STATE how many of each atom are in the molecule. Ionic or Covalent? There are 4 good methods to tell if something is Ionic or Covalent. ◦ Electronegativity Difference of 1.9 to 0 is a covalent bond. ◦ If the first element is on the top ½ of the ion sheet, then its IONIC. Likewise, if its NOT on the top ½ of the list, it’s COVALENT. ◦ Metal plus non-metal is IONIC, and non-metal plus non-metal is COVALENT. ◦ If an element from the right side combines with an element from the right, it’s IONIC. Covalent Nomenclature If the first element is singular, then use the whole name of the element and use prefixes for the second element and change the ending to “ide” CO = Carbon Monoxide CO2 = Carbon Dioxide NO = Nitrogen Monoxide NO2 = Nitrogen Dioxide Covalent Nomenclature If the first element in a covalent molecule is multiple, then we must include a PREFIX. i.e. P3O7 is named as ___? Triphosphorus heptaoxide N4O9 is named as_________? Tetranitrogen Nonaoxide H2O is commonly known as water, but what is the proper name? Dihydrogen monoxide Moles to Number of Particles 23 1 mol = 6.022 x 10 particles 6.022 x 1023 particles / 1 mol = 1 Molar Mass The mass in grams of 1 mole of a substance Equal to atomic mass 1 mole = atomic mass of an element