printer-friendly sample test questions
advertisement

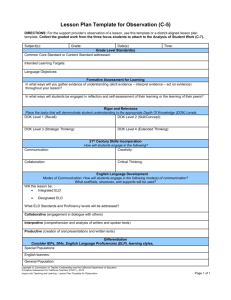
Content Benchmark P.12.A.3 Students know identifiable properties can be used to separate mixtures. 1st Item Specification: Distinguish between mixtures and compounds. Depth of Knowledge Level 1 1. Which of the following is considered to be a pure substance? A. Granite. B. Sodium chloride. C. Muddy water. D. Milk of Magnesia. 2. Physical properties of a mixture A. vary with the amount of substance. B. depend on the volume of the substance. C. depend on the organization of the substance. D. vary depending upon its components. 3. Examples of physical properties that depend on the quantity of a substance include A. boiling point, color. B. color, density. C. melting point, solubility. D. volume, mass. 4. Mixtures A. can have the same composition throughout. B. have physical properties dependent on its components. C. have the same physical properties as compounds. D. cannot be separated into their components. 5. Compounds A. are the same as mixtures. B. can be separated by their physical properties. C. contain only one type of element. D. are different kinds of atoms chemically combined with each other. Depth of Knowledge Level 2 6. White gold is used in jewelry and contains two elements, gold and palladium. A jeweler has two different samples that are both identical in appearance and have a uniform composition throughout. What can be said about the samples? A. They are homogeneous mixtures and be classified as metallic alloys. B. The materials are heterogeneous mixtures and can be classified by their components. C. The samples have variable compositions and are classified as metallic solutions. D. The samples are heterogeneous mixtures that can be separated using magnetic properties. 7. A student is given an unknown substance and asked to perform paper chromatography to determine whether it is a mixture or a pure substance. She placed several drops of the substance on the paper. Her results are below. (From http://www.chemmybear.com/groves/apchem.html) She concludes that A. she has a mixture because multiple bands of color are evident. B. she has one substance only because the top of the paper is blue. C. the experiment is inconclusive because the bands are different. D. chromatography is not a good method of separating substances. 8. When a pure solid substance was heated, it changed into another solid and a gas, each of which was a pure substance. Based on these observations, the original solid A. was not a single element. B. was a compound. C. is a compound and the products are elements. D. was a mixture of two other substances. 9. A brass alloy contains 66% copper and 34% zinc. It is best described as A. a pure substance because it contains blended elements. B. a compound because it contains two different types of atoms. C. a solution because it has the same composition throughout. D. separable because copper and zinc have different conductivities. 2nd Item Specification: Distinguish between heterogeneous and homogeneous mixtures. Depth of knowledge Level 1 10. Which of the following is an example of a heterogeneous substance? A. Bottled water B. Table salt C. Pieces of copper D. Candle 11. Which of the following is an example of a heterogeneous substance? A. Calcium carbonate B. Sodium sulfate C. Aspirin D. Salad dressing 12. Which of the following is an example of a heterogeneous substance? A. Teflon B. M&M candy C. Vitamin water D. Red Kool-Aid beverage 13. Which of the following is an example of a homogeneous substance? A. Granite B. Table salt C. M&M candy D. Muddy water 14. Which of the following is an example of a homogeneous substance? A. Glass B. Dirt C. Flowers D. Ice cream sundae 15. Which of the following is an example of a homogeneous substance? A. Green tea B. Sandwich C. Chocolate Chip Cookie D. Pine cone Depth of Knowledge Level 2 16. Which of the following contains a homogeneous mixture? A. B. C. D. Sample A Sample B Sample C Sample D 17. Which of the following diagrams shows a heterogeneous mixture? (From http://www.edb.utexas.edu/insite/users/mthiessen/studyguides/final_exam_study_guide.htm) A. B. C. D. Diagram a, b and c Diagram a and c Diagram b, c and d Diagram a and b 18. Which of the following shows the correct example of a heterogeneous material? A. Mixture same composition throughout solution. B. Mixture magnet two separate substances. C. Mixture filtration one substance. D. Air wind one solution of gases. 19. Which flow chart correctly describes a homogeneous material? A. Unknown density 3 layers. B. Unknown filtration two substances. C. Unknown magnet two substances. D. Unknown boiling one temperature. 3rd Item Specification: Design separation processes based on properties (e.g., magnetism, solubility, density, boiling point, and properties that lend themselves to mechanical sorting). Depth of Knowledge Level 1 20. Filtration can be used to separate A. solids from solids. B. liquids from solids. C. liquids from liquids. D. liquids from gases. 21. One common method used to separate dyes is A. filtration. B. distillation. C. chromatography. D. conductivity. 22. Melting points can separate materials because A. substances melt at different temperatures. B. molecules vibrate rapidly when heated. C. heat causes molecules to disintegrate. D. many substances fuse at the melting point. 23. Distillation is a good separation technique for A. solids. B. liquids. C. solid alloys. D. gases. 24. Solubility is a good separation technique for A. pure metals. B. noble gases. C. different salts. D. metallic alloys. 25. Magnetism is most beneficial for separating A. gases and non-metallic liquids. B. magnetic solids and solids such as sulfur. C. non-metallic solids and solids such as sulfur. D. non-magnetic solids from non-magnetic liquids. Depth of Knowledge Level 2 26. The following graph was made of two liquid being distilled. What observation can be made regarding point B? T 0C Heat Added What observation can be made regarding point B? A. The first liquid component is boiling. B. The second component has already boiled. C. The mixture is homogeneous. D. Energy is being removed from the liquid. 27. A student has three unknown solid metals. Which series of tests would be the MOST appropriate to use to distinguish among the metals? A. Boiling point, density and color. B. Color, density and mass. C. Density, mass and volume. D. Conductivity, density and color. 28. A student has a mixture of sand, water, salt and iron pieces. Which procedure would separate the mixture? A. Evaporate the water, use a magnet to remove the iron. B. Filter the water to separate the sand, use a magnet to remove the iron. C. Filter the sand and iron from the water, use a magnet to remove the iron from the sand, evaporate the water to separate the salt. D. Filter the sand and iron from the water; evaporate the water to separate the salt. 29. Which of the following observations could be used to separate two metals? A. Bubbles form when the substance is dropped into water. B. The color of both metals is grey. C. One metal is attracted to a magnet. D. The volumes of the metals are different. Content Benchmark P.12.A.3 Students know identifiable properties can be used to separate mixtures. Answers to Sample Test Questions 1. B, DOK Level 1 2. D, DOK Level 1 3. D, DOK Level 1 4. B, DOK Level 1 5. D, DOK Level 1 6. A, DOK Level 2 7. A, DOK Level 2 8. B, DOK Level 2 9. C, DOK Level 2 10. D, DOK Level 1 11. D, DOK Level 1 12. B, DOK Level 1 13. B, DOK Level 1 14. A, DOK Level 1 15. A, DOK Level 1 16. D, DOK Level 2 17. A, DOK Level 2 18. B, DOK Level 2 19. D, DOK Level 2 20. B, DOK Level 1 21. C, DOK Level 1 22. A, DOK Level 1 23. B, DOK Level 1 24. C, DOK Level 1 25. B, DOK Level 1 26. A, DOK Level 2 27. D, DOK Level 2 28. C, DOK Level 2 29. C, DOK Level 2