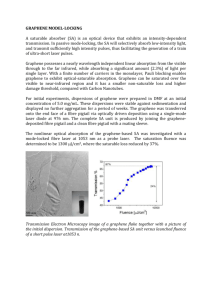
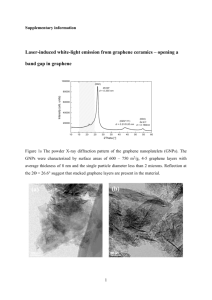
srep01415
advertisement

1 Laser Engineered Graphene Paper for Mass Spectrometry Imaging Supplementary Information Kun Qian,1 Liang Zhou,1 Jian Liu,1 Jie Yang,1 Hongyi Xu,2 Meihua Yu,1 Amanda Nouwens,3 Jin Zou,2 Michael J. Monteiro1 and Chengzhong Yu1* 1 Australian Institute for Bioengineering and Nanotechnology, 2Materials Engineering and Centre for Microscopy and Microanalysis, and 3School of Chemistry and Molecular Biosciences, the University of Queensland, Brisbane, QLD 4072, Australia. E-mail: c.yu@uq.edu.au; Fax: (+) 61-7-33463973 This part includes: Supplementary Methods Table S1 Figure S1 to S10 2 Supplementary Methods: Synthesis of the graphene paper. In the preparation of the graphene sheets, a chemical oxidation-reduction approach was adapted, where the graphite was oxided by the Staudenmaier method.1 5.0 g of graphite was added into a mixture solution of concentrated sulphuric acid (87.5 mL) and fuming nitric acid (45 mL) and cooled in an ice-water bath. Then 55 g of KClO3 was added very slowly with a period longer than 15 min into the mixture. All the operations were carried out very carefully in a fume hood to reduce the risk of explosion due to the release of chlorine dioxide gas. After stirring for 96 h at room temperature, the mixture was poured into 4 L of water, filtered and washed to obtain graphite oxides. The as-made graphite oxide (0.30 g) was dispersed into a mixed solvent (150 ml of ethylene glycol, 75 ml of water and 75 ml of PEG2000), then sonicated (SCIENTZ SB 3200DTN, 150W) for 2 hours to form a homogeneous graphene oxide suspension. Then the whole suspension was transferred into a Teflon-lined stainless steel autoclave and reacted to perform solvent thermal reduction at 180 ºC for 24 hours.2, 3 Finally the graphene paper was fabricated by vacuum filtration of 50 mL of reacted mixture. 1. 2. 3. Hontorialucas, C., Lopezpeinado, A.J., Lopezgonzalez, J.D.D., Rojascervantes, M.L. & Martinaranda, R.M. STUDY OF OXYGEN-CONTAINING GROUPS IN A SERIES OF GRAPHITE OXIDES - PHYSICAL AND CHEMICAL CHARACTERIZATION. Carbon 33, 1585-1592 (1995). Huang, X.D. et al. A magnetite nanocrystal/graphene composite as high performance anode for lithium-ion batteries. J. Alloy. Compd. 514, 76-80 (2012). Huang, X.D. et al. A Facile One-Step Solvothermal Synthesis of SnO2/Graphene Nanocomposite and Its Application as an Anode Material for Lithium-Ion Batteries. ChemPhysChem 12, 278-281 (2011). 3 Table S1 Comparison of the typical laser engineering techniques towards the graphene film or paper Laser Wavelength Laser Operation Spot Size Application Reference Source (nm) Power Model (µm) Field Source DVD 788 nm 5 mW Continuous Not Given Capacitors Ref1 Laser Infrared 2.4 W Continuous ~ 100 µm Capacitors Ref2 Printer (CO2 laser) Smart 337 nm 20 mW Pulsed, 200 Hz ~ 30 µm MS Detection This work Beam (N2 laser) Drives 1 (3 ns width) and Imaging El-Kady, M.F., Strong, V., Dubin, S. & Kaner, R.B. Laser Scribing of High Performance and Flexible Graphene- Based Electrochemical Capacitors. Science 335, 1326-1330 (2012). 2 Gao, W. et al. Direct laser writing of micro-supercapacitors on hydrated graphite oxide films. Nat. Nanotechnol. 6, 496-500 (2011). 4 Figure S1. Schematics for the chemo-synthesis of graphene sheets from graphite. The graphite was first oxidized to graphite oxide using the Staudenmaier method, which was exfoliated to graphene oxide by sonication. The graphene sheets were obtained through reducing the graphene oxide by a solvent thermal treatment method. 5 Figure S2. X-ray photoelectron spectroscopy characterisation of the graphene paper before and after laser engineering. (a) Untreated graphene paper. (b) Engineered paper with 50% laser intensity. 6 Figure S3. Low magnification SEM characterisation of the graphene paper engineered at different laser intensities. (a) Untreated graphene paper. (b) 10% laser intensity. (c) 30% laser intensity. (d) 50% laser intensity. The scale bar is 10 µm for each image and the circled area stands for the typical area for further high resolution SEM observations. 7 Figure S4. SEM images of the engineered graphene paper after sonication. (b) is the high resolution image of the nanospheres in (a). 8 Figure S5. Matrix-free LDI MS detection of AONB on commercial NALDI™ plate and engineered graphene paper. MS spectra of 0.85 fmol of AONB on (a) commercial NALDI™ substrate and (b) engineered graphene paper. Asterisks indicate the identified ions from the molecules. 9 Figure S6. Matrix-assisted LDI MS detection of AONB and DOTAP lipid on the commercial MALDI plate. MALDI MS spectra of (a) 8.5 fmol, (b) 0.85 fmol of AONB and (c) 300 fmol, (d) 30 fmol of DOTAP on the commercial MALDI plate using CHCA as matrix. Asterisks indicate the identified ions from the molecules. 10 Figure S7. Matrix-free LDI MS detection of DOTAP lipid on engineered graphene paper. MS spectrum of 30 fmol DOTAP lipid on engineered paper and asterisks indicate the identified ions from the lipid molecules. 11 Figure S8. Matrix-free LDI MS detection of DPPC and HSPC lipids on the untreated and engineered graphene paper. MS spectra of (a & b) 2.7 pmol DPPC, (c & d) 2.6 pmol HSPC on untreated and engineered graphene paper, respectively. MS spectra of (e) 270 fmol DPPC lipid and (f) 260 fmol HSPC lipid on the engineered graphene paper. Asterisks indicate the identified ions from the lipid molecules. 12 Figure S9. The digital image of the stamped paper. The central dark black area (laser engineered area) has been stamped with the letters. The stamp was made by laser engraving the letters. The size of the stamp is 1 cm X 1 cm of all and the size of the letters on the stamp is ~ 2 mm in height. 13 Figure S10. MS imaging patterns of the letters. 'A' at m/z of 662 and 380 on (a, b) untreated graphene paper and (c, d) engineered graphene paper, respectively. 'B' at m/z of 662 and 380 on (e, f) untreated graphene paper and (g, h) engineered graphene paper, respectively. The scale bar in a-h is 500 µm.