Efferdent

Name ____________________________________________________ Date _______________
Lab: Efferdent
Objective:
To observe the speed of a reaction be changed.
To state the relationship between surface area, temperature and reaction time.
Background:
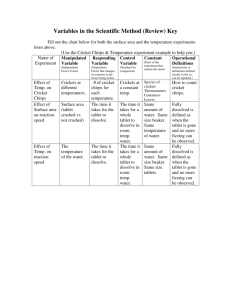
All chemical reactions begin with reactants and end with products . The reactants are the starting materials and the products are the result of the reaction between reactants. Every chemical reaction has a rate or a speed of how quickly the reactants will turn into products.
Reaction rates depend on a number of factors. In this lab we will study surface area, and temperature . Below is the chemical equation for this experiment. Efferdent is a source of hydrogen peroxide. The symbol for Hydrogen peroxide is
H
2
O
2.
H
2
O
2
+ catalyst ---> _________ + H
2
O
Questions #1: What are the reactants in this lab?
Questions #2: What are the products in this lab?
Questions #3: What do we use as the catalyst in this lab?
Efferdent is a compound that when placed in water begins to fizzle and a color change can be observed. The job of Efferdent is to clean dentures by reducing plaque buildup and removing food particles that can build up on dentures.
Materials: 2 250-ml beakers, 3 Efferdent tablets, hot and cold water,
Procedure:
1.
Fill one beaker each with 200 ml of tap water and the other beaker with hot water.
2.
Drop one tablet into each beaker and make observations every minute for 10 minutes.
Record all data in data tables II, and I and be sure to include any changes in the size of the tablet .
3.
Next crush the last tablet into a powder and drop it into 200 ml of tap water. Again, make observations for 10 minutes and record this data into data table III also .
5
6
3
4
Observation:
Time Tap Water
1
2
Hot Water Crushed Tablet
7
8
9
10
Analysis:
1.
Which type of water (warm or cold) dissolved the tablet the quickest?
2.
From what we learned what type of change is indicative by the color change?
3.
From what we learned what type of change is indicative of the bubbling?
4.
If you were to compare the crushed tablet to the whole tablet placed in cold water, which one dissolved in the shortest amount of time?
5.
In this experiment the mass of the water is much more than the mass of the tablet, therefore, the concentration of the water is higher than the concentration of the tablet. If the mass of the water was equal to the mass of the tablet, what do you think would happen?
6.
Based on your answer to the questions above, how do you think the amount of solvent impacts the speed of a reaction?
Conclusion:
In at least five sentences discuss what you learned in this lab. In your conclusion make sure you include a discussion of the following:
Effect of temperature on reaction time between the tablet and the water?
Effect of change in particle size of one of the reactants; and its effect upon the speed of the chemical reaction