Reaction Rates: Particle Size & Temperature Worksheet
advertisement

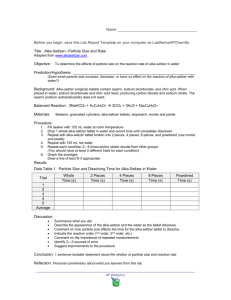
Name _________________________ Particle Size (Surface Area), temperature and Rate of Reaction Ideas The rate of a chemical reaction is affected by the physical size of the reactants and by temperature. Decreasing the size of the particles which make up a given weight will increase the number of particles represented by the same weight. Smaller particle size results in an increase in the rate of reaction. Similarly, warmer water (or conditions) will speed up a reaction. My predictions: 1.) The powder will react _______ times faster than the whole tablet. 2.) Using hot water out of the tap, the rate of reaction will be _________ times faster than with cold water. Materials Note: All experiments should be done using original formula effervescent Alka-Seltzer. 2 Clear beakers 3 Alka-Seltzer tablets Mortar and pestle (pencil and sheet of paper) Stopwatch (on iPad) Procedure I. Particle Size A. Whole Tablet 1. Fill a clear glass beaker with exactly 100 ml of cold water. 2. Drop 1 whole Alka-Seltzer tablet into the water. Measure and record the time to dissolve. B. Powdered Tablet 1. Place 1 Alka-Seltzer tablet on to a sheet of paper and grind to a fine powder. 2. Transfer powder into a clear cup. (Note: It's important to have the powder in the cup before adding water.) 3. Add 100 ml of cold water to the glass. Measure and record the time to dissolve. Name _________________________ Observations Particle Size Time for reaction to be completed Whole Tablet _________ Seconds Powder _________Seconds Summary a) As particle size decreases, the rate of reaction _______________. b) The rate of reaction for the powder was ___________ times faster than for the whole tablet. Questions 1. As particle size decreases, the total surface area of a reactant ________________. As a result, the probability of interactions between atoms/ions ________________, and the rate of reaction ___________________. 2. Particle size appears to have _______________ (less or more) of an effect on the rate of reaction than temperature. II. Water Temperature A. Hot Water 1. Run water from the hot tap until it is as hot as possible. Fill a clear glass beaker with exactly 100ml of hot water. 2. Remove 1 Alka-Seltzer tablet from its package. Drop it into water. Measure the time required for tablet to fully dissolve. Be prepared to start and stop on time. The reaction may take less than 15 seconds. Record the time. B. Cold Water 1. Use your data from the whole, cold tablet in part one, when comparing with the hot water tablet you just conducted. Observations Time for Reaction to be Completed Hot Tap _____ Seconds Cold Water _____ Seconds Name _________________________ Summary As the temperature increases, the rate of reaction _____________________________. Questions 1. Using hot tap water, the rate was ______ times faster than at 0 degrees C. III. My thoughts Explain why smaller particle size allows for a faster reaction. ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ Explain why warmer temperature allows for a faster reaction. ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________